Abstract
Background
As a new obesity-related index, the weight-adjusted-waist index (WWI) appears to be a good predictor of cardiovascular disease (CVD) in East Asian populations. This study aimed to validate the association between WWI and CVD in United States (US) adults and also evaluate its relationships with the prevalence of specific CVDs.
Methods
The data were obtained from the 2009–2016 National Health and Nutrition Examination Survey. WWI was calculated as waist circumference divided by the square root of weight, and CVD was ascertained based on self-reported physician diagnoses. Multivariable logistic regression models and subgroup analyses were performed to evaluate the association between WWI and CVD.
Results
A total of 21,040 participants were included. There was a positive linear relationship between WWI and the odds of CVD (P = 0.310). After adjusting for all covariates, each unit of increased WWI was associated with 48% increased risk of CVD (odds ratio [OR]: 1.48, 95% confidence interval [CI]: 1.25–1.74). Moreover, compared with the lowest quintile (< 10.3 cm/√kg), the multivariable-adjusted OR was 3.18 (95% CI: 1.80–5.59) in the highest quintile (≥ 11.8 cm/√kg). Besides, positive associations were also found between WWI and increased prevalence of congestive heart failure (OR: 1.47, 95% CI: 1.11–1.96), coronary heart disease (OR: 1.27, 95% CI: 1.01–1.60), angina (OR: 1.44, 95% CI: 1.06–1.96), heart attack (OR: 1.66, 95% CI: 1.29–2.12), and stroke (OR: 1.32, 95% CI: 1.02–1.70). Subgroup analyses showed that stronger associations between WWI and CVD were detected in participants younger than 50 years of age (P < 0.001).
Conclusions
High levels of WWI were significantly associated with an increased risk of CVD in US adults, particularly in people under 50 years of age. These findings indicate that WWI may be an intervention indicator to reduce the risk of CVD in the general adult population.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-023-03452-z.
Keywords: Weight-adjusted-waist index, Obesity, Cardiovascular disease, NHANES
Background
Obesity is a major public health problem worldwide. The global prevalence of obesity has doubled since 1980, and it is expected to reach 18% in men and surpass 21% in women by 2025 [1, 2]. According to statistics from the National Health and Nutrition Examinations Survey (NHANES) 2015–2016, approximately 39.6% of adults and 18.5% of youths were obese in the United States (US) [3]. Furthermore, large-scale and long-term studies have consistently demonstrated that obesity is associated with a significantly increased risk of cardiovascular disease (CVD) morbidity and mortality [4, 5]. Therefore, early identification of obese individuals with high risk is crucial to preventing CVD.
Body mass index (BMI) and waist circumference (WC) are the most commonly used obesity-related indices. Accumulating data has indicated that they are strongly associated with a rise in the prevalence of hypertension, stroke, and other CVDs [6, 7]. However, in recent years, controversy regarding the health outcomes of overweight and obesity has grown in CVD patients, given findings of similar or lower all-cause mortality compared with their normal-weight counterparts [8–10]. This is partly because these anthropometric measures do not clearly distinguish between muscle mass and fat mass [11, 12]. Although the waist-to-height ratio (WHtR) appears to be superior in assessing obesity, it remains controversial in predicting obesity-related CVD risk and mortality [13, 14]. As a result, these traditional indices may not accurately reflect the relationship between obesity and CVD risk.
In this context, a new anthropometric index called the weight-adjusted-waist index (WWI) was proposed, which standardized WC for body weight. Subsequently, Park et al. found that in the Korean National Cohort study, WWI was a better predictor of CVD mortality than BMI, WC, and WHtR. Also, only WWI demonstrated a linear positive correlation between adiposity indices and cardiovascular mortality, but not BMI, WC, or WHtR [15]. Additionally, several prospective studies in China discovered that higher WWI levels were associated with an increased risk of all-cause and cardiovascular mortality [16, 17]. Unfortunately, the associations were only validated in East Asian populations. Moreover, there is relatively little information on the relationships between WWI and the prevalence of specific CVDs (such as coronary heart disease and stroke).
To address the knowledge gap, this study aimed to investigate the associations of WWI, an innovative anthropometric index, with CVD and its subtypes in US adults based on data obtained from the NHANES.
Methods
Study design
NHANES is an ongoing cross-sectional survey administered by the National Center for Health Statistics (NCHS), which is part of the Centers for Disease Control and Prevention (CDC), to assess the health and nutritional status of adults and children in the United States. It documents a repeated two-year cycle with five major parts, including demographic data, dietary data, examination data, laboratory data, and questionnaire data. Due to the multi-stage, stratified, and probability sampling design, the included participants showed relatively great representativeness. The details of NHANES study design and methods have been previously described [18]. The NHANES study protocol was approved by the NCHS Research Ethics Review Board, and written informed consent was obtained from each participant.
Study population
We used the continuous NHANES data from 2009 to 2016 (N = 40,439). Participants younger than 18 years of age (N = 15,943) and those with incomplete CVD evaluation data (N = 1,231) were excluded. Then, we further excluded participants with missing data on weight and WC value (N = 2,225). Finally, a total of 21,040 participants were included for current analysis. The detailed flow chart of participant selection is shown in Additional File Fig. 1. All data included in this manuscript are publicly available at https://www.cdc.gov/nchs/nhanes/.
Covariates of interest
Potential covariates, including demographic data (age, gender, educational level, and race/ethnicity), lifestyle variables (smoking status and alcohol drinking), anthropometric measurements (height, weight, WC, and blood pressure [BP]), and laboratory results (hemoglobin A1c [HbA1c], total bilirubin [TBIL], low-density lipoprotein cholesterol [LDL-C], high-density lipoprotein cholesterol [HDL-C], total cholesterol [TC], triglycerides [TG], serum creatinine [Scr], serum uric acid [SUA], and urinary albumin/creatinine ratio [UACR]) were selected based on clinical relevance and statistical significance. Demographics and lifestyle data were derived from the household interview questionnaires administered by highly trained medical personnel. Anthropometric indicators and biochemical parameters were obtained through medical examinations and subsequent laboratory assessments in the Mobile Examination Centre (MEC).
The educational level was further categorized as less than 9th grade, 9-11th grade, high school graduate, some college or AA degree, and college graduate or above. Race/ethnicity was classified as Mexican American, other Hispanic, non-Hispanic black, non-Hispanic white, and Other Race (including multi-racial). The smoking status was determined by “Smoked at least 100 cigarettes in life”, and the alcohol drinking status was evaluated by “Had at least 12 alcohol drinks per year”. The history of asthma and cancer was examined through the item “Ever been told you have asthma and/or cancer or malignancy”. The laboratory results, including HbA1c, TBIL, LDL-C, HDL-C, TC, TG, Scr, SUA and UACR levels were determined using standardized methods. The detailed measurement processes of these variables are publicly available at https://www.cdc.gov/nchs/nhanes/. Additionally, the BMI in kg/m2 was calculated by dividing weight (kg) by the square of height (m2), and the WHtR was determined by WC (cm) divided by height (cm). The estimated glomerular filtration rate (eGFR) was calculated using the recently published Chronic Kidney Disease Epidemiology Collaboration creatinine equation [19].
Exposure variable and outcomes
In this study, WWI (cm/√kg) was designed as exposure variable. WWI was developed according to the formula [In (WC) = β0 + β1 In (weight) + ε]. Since the estimated β1 was 0.494 (close to 0.5), the final formula of WWI was calculated as WC (cm) divided by the square root of weight (kg) [15]. Weight was measured to the nearest 0.1 kg using a digital weight scale, and WC was measured by a retractable steel measuring tape, positioning the measuring tape around the waist at the uppermost lateral border of the ilium at the midaxillary line. The full procedure, including the protocols, equipment, and quality control, was described at https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/manuals.aspx?BeginYear=2009.
The outcome variable was CVD. According to previous studies, CVD was defined as a composite of 5 self-reported cardiovascular outcomes, which included congestive heart failure (CHF), coronary heart disease (CHD), angina/angina pectoris, heart attack, and stroke [20]. All participants were asked the following questions: “Has a doctor or other health professional ever told you that you have congestive heart failure/coronary heart disease/angina pectoris/heart attack/stroke? ” Participants who answered “yes” to any of the questions were considered to have CVD. Additionally, we also collected data for each CVD subtype to further analyze the association with WWI.
Statistical analysis
All statistical analyses were performed in accordance with NHANES analytic guidelines [21]. Four waves of continuous survey data (NHANES 2009–2010, 2011–2012, 2013–2014, and 2015–2016) were combined, and an 8-year sampling weight was calculated by using a quarter of the 2-year sampling weight (WTMEC2YR).
Data are presented as weighted mean ± standard deviation (SD) or median (interquartile range, IQR) for continuous variables, and frequency (weighted percentage) for categorical variables. Comparisons between the CVD and non-CVD groups were performed using either the weighted Chi-square test (categorical variables) or the weighted linear regression model (continuous variables). In sensitivity analysis, WWI was converted from a continuous variable to a categorical variable (quintiles) to evaluate its robustness. Multivariable logistic regression model was used to calculate odds ratios (ORs) and corresponding 95% confidence intervals (CIs) to determine the prevalence of CVD related to WWI. We developed three models to adjust for potential confounders: model 1 was a crude model; model 2 was adjusted for age, gender, and race; model 3 was the same as model 2 with additional adjustment for education level, smoking, alcohol drinking, eGFR, systolic and diastolic BP, HbA1c, TBIL, LDL-C, HDL-C, TC, TG, Scr, SUA and UACR. The restricted cubic spline model was used for the dose-response analysis between WWI and total CVD. Subgroup analysis stratified by gender, age, race, BMI (< 25 / 25–30 / ≥ 30 kg/m2), WC (abnormal: WC ≥ 94 cm in male and WC ≥ 80 cm in female), eGFR (< 90 / ≥ 90 ml/min/1.73m2), smoking, and alcohol drinking was conducted by stratified multivariate regression analysis. A receiver operating characteristic (ROC) curve was used to analyze the predictive value of WWI and traditional obesity-related indices (BMI, WC, and WHtR) for CVD. Besides, we used multiple imputation (MI), based on 5 replications and the Markov-chain Monte Carlo method in the SAS MI procedure, to account for missing data on BMI, systolic BP, diastolic BP, alcohol consumption, TG, and LDL-C (Model 3).
All analyses were performed using R version 4.0.3 (www.R-project.org) and EmpowerStates (www.empowerstats.com). A two-sided P value of < 0.05 was considered statistically significant.
Results
Baseline characteristics
The characteristics of the study population are presented in Table 1. A total of 21,040 participants were included in this study, 51.47% of whom were females, with an average age of 47.11 ± 16.79 years. The weighted mean WWI was 10.98 ± 0.83 cm/√kg overall, and the weighted prevalence of total CVD, CHF, CHD, angina, heart attack, and stroke were 7.91% (N = 2063), 2.20% (N = 599), 3.21% (N = 794), 1.96% (N = 481), 3.12% (N = 810) and 2.47% (N = 691), respectively. Compared with the non-CVD group, the CVD group was older and more likely to be male; to have higher BMI, WC, WHtR, WWI, SBP, HbA1c, TG, Scr, SUA, and UACR levels; to have a higher proportion of smoking and non-Hispanic White individuals; to have a lower rate of alcohol drinking; and to have lower eGFR, LDL-C, and HDL-C levels (all P < 0.05).
Table 1.
Baseline characteristics of the study participants
| Variables# | Total (n = 21,040) |
Non-CVD (n = 18,977) |
CVD (n = 2063) |
P value |
|---|---|---|---|---|
| Age (years) | 47.11 ± 16.79 | 45.65 ± 16.25 | 64.15 ± 13.32 | < 0.001 |
| Gender (%) | < 0.001 | |||
| Male | 10,243 (48.53) | 9059 (47.93) | 1184 (55.46) | |
| Female | 10,797 (51.47) | 9918 (52.07) | 879 (44.54) | |
| Race (%) | < 0.001 | |||
| Mexican American | 3153 (8.65) | 2941 (8.96) | 212 (5.04) | |
| Other Hispanic | 2263 (5.97) | 2078 (6.14) | 185 (4.08) | |
| Non-Hispanic White | 8505 (66.26) | 7449 (65.73) | 1056 (72.50) | |
| Non-Hispanic Black | 4424 (11.12) | 3973 (11.06) | 451 (11.70) | |
| Other Race | 2695 (8.01) | 2536 (8.12) | 159 (6.68) | |
| Educational level (%) | < 0.001 | |||
| < 9th grade | 2135 (5.45) | 1832 (5.16) | 303 (8.83) | |
| 9−11th grade | 2915 (10.56) | 2551 (10.22) | 364 (14.57) | |
| High school | 4630 (21.28) | 4114 (20.86) | 516 (26.18) | |
| College | 6273 (32.09) | 5715 (32.18) | 558 (30.98) | |
| Graduate or above | 5070 (30.57) | 4750 (31.53) | 320 (19.41) | |
| BMI (kg/m2) | 28.94 ± 6.74 | 28.79 ± 6.68 | 30.65 ± 7.25 | < 0.001 |
| WC (cm) | 99.20 ± 16.38 | 98.57 ± 16.21 | 106.57 ± 16.57 | < 0.001 |
| WHtR | 0.59 ± 0.10 | 0.59 ± 0.10 | 0.64 ± 0.10 | < 0.001 |
| WWI (cm/√kg) | 10.98 ± 0.83 | 10.93 ± 0.82 | 11.55 ± 0.75 | < 0.001 |
| Smoking (%) | 9183 (44.00) | 7933 (42.46) | 1250 (61.91) | < 0.001 |
| Alcohol drinking (%) | 13,935 (77.98) | 12,579 (78.38) | 1356 (73.57) | < 0.001 |
| SBP (mmHg) | 122.11 ± 17.17 | 121.49 ± 16.75 | 129.32 ± 20.04 | < 0.001 |
| DBP (mmHg) | 70.52 ± 12.17 | 70.76 ± 11.96 | 67.66 ± 14.11 | < 0.001 |
| eGFR (ml/min/1.73m2) | 118.05 ± 46.03 | 120.31 ± 45.50 | 91.71 ± 43.90 | < 0.001 |
| HbA1c (%) | 5.63 ± 0.93 | 5.59 ± 0.88 | 6.17 ± 1.25 | < 0.001 |
| TBIL (umol/L) | 11.49 ± 5.24 | 11.48 ± 5.22 | 11.71 ± 5.44 | 0.082 |
| HDL-C (mmol/L) | 1.39 ± 0.44 | 1.39 ± 0.44 | 1.29 ± 0.43 | < 0.001 |
| LDL-C (mmol/L) | 2.95 ± 0.91 | 2.99 ± 0.90 | 2.55 ± 0.88 | < 0.001 |
| TC (mmol/L) | 5.01 ± 1.08 | 5.04 ± 1.07 | 4.63 ± 1.09 | < 0.001 |
| TG (mmol/L) | 1.40 ± 1.18 | 1.38 ± 1.17 | 1.57 ± 1.19 | < 0.001 |
| Scr (umol/L) | 77.91 ± 30.25 | 76.56 ± 27.31 | 93.57 ± 51.11 | < 0.001 |
| SUA (umol/L) | 321.45 ± 83.23 | 318.95 ± 82.03 | 350.49 ± 91.20 | < 0.001 |
| UACR (mg/g) | 7.05 (4.55, 13.33) | 6.78 (4.46, 12.22) | 11.82 (6.16, 33.19) | < 0.001 |
| Asthma (%) | 3063 (15.04) | 2630 (14.46) | 433 (21.85) | < 0.001 |
| Cancer or malignancy (%) | 1945 (10.24) | 1505 (9.14) | 440 (23.09) | < 0.001 |
# Values are presented as weighted mean ± standard deviation, medians (interquartile range), or frequency (weighted percentages) when appropriate. Abbreviations: CVD, cardiovascular disease; BMI, body mass index; WC, waist circumference; WHtR, waist-to-height ratio; WWI, weight-adjusted-waist index; SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; TBIL, total bilirubin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides; Scr, serum creatinine; SUA, serum uric acid; UACR, urinary albumin/creatinine ratio.
Association of WWI with CVD
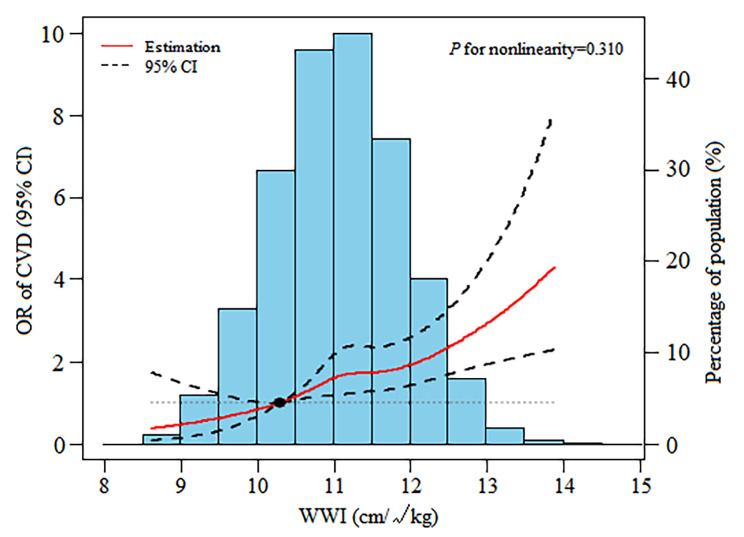
Multivariable logistic regression models were performed to explore the association between CVD and the WWI as continuous and categorical variables (Table 2). When WWI was analyzed as a continuous variable, we found that increased WWI was associated with a higher risk of CVD (Model 1: OR = 2.50, 95% CI: 2.33–2.69; Model 2: OR = 1.74, 95% CI: 1.58–1.92; all P < 0.001). In the fully adjusted Model 3, the results indicated that each unit of increased WWI was associated with 48% increased risk of CVD (OR = 1.48, 95% CI: 1.25–1.74, P < 0.001). In sensitivity analysis, the multivariable-adjusted ORs (reference to Quintile 1) was 1.74 (95% CI: 1.00-3.02; P = 0.048) for Quintile 2, 2.38 (95% CI: 1.38–4.13; P = 0.002) for Quintile 3, 2.56 (95% CI: 1.47–4.45; P < 0.001) for Quintile 4, and 3.18 (95% CI: 1.80–5.59; P < 0.001) for Quintile 5, indicating a stable positive association between higher WWI and increased risk of CVD (P for trend < 0.001). Moreover, we reanalyzed the association between WWI and CVD using imputation data and the results did not change qualitatively (Additional File Table 1). In addition, the restricted cubic spline with a multivariate logistic regression model revealed that there was a positive linear relationship between WWI and the odds of CVD (P for nonlinear = 0.310) (Fig. 1).
Table 2.
Adjusted odds ratios (95% CI) for association between WWI and the prevalence of CVD.
| WWI (cm/√kg) | Events (%) |
OR (95% CI), P value | ||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||
| Per 1 cm/√kg increase | 2063 (7.91) | 2.50 (2.33, 2.69) < 0.001 | 1.74 (1.58, 1.92) < 0.001 | 1.48 (1.25, 1.74) < 0.001 |
| Categorical | ||||
| Quintile 1 (< 10.3) | 108 (1.75) | Reference | Reference | Reference |
| Quintile 2 (10.3–10.8) | 215 (3.95) | 2.31 (1.69, 3.14) < 0.001 | 1.42 (1.04, 1.96) 0.027 | 1.74 (1.00, 3.02) 0.048 |
| Quintile 3 (10.8–11.3) | 391 (7.96) | 4.85 (3.64, 6.45) < 0.001 | 2.25 (1.67, 3.04) < 0.001 | 2.38 (1.38, 4.13) 0.002 |
| Quintile 4 (11.3–11.8) | 560 (11.19) | 7.06 (5.36, 9.30) < 0.001 | 2.65 (1.96, 3.58) < 0.001 | 2.56 (1.47, 4.45) < 0.001 |
| Quintile 5 (≥ 11.8) | 789 (17.57) | 11.94 (9.12, 15.63) < 0.001 | 3.87 (2.86, 5.22) < 0.001 | 3.18 (1.80, 5.59) < 0.001 |
| P for trend | < 0.001 | < 0.001 | < 0.001 | |
Model 1: crude model;
Model 2: adjusted for age, gender and race;
Model 3: adjusted for age, gender, race, education level, smoking, alcohol drinking, systolic blood pressure, diastolic blood pressure, estimated glomerular filtration rate, hemoglobin A1c, total bilirubin, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, total cholesterol, triglycerides, serum creatinine, serum uric acid, and urinary albumin/creatinine ratio. The covariates were determined based on the matched odds ratio changed at least 10% when added to this model. Abbreviations: WWI, weight-adjusted-waist index; CVD, cardiovascular disease; OR, odds ratio; CI, confidence interval.
Fig. 1.
Dose-response relationship between WWI and the risk of CVD. Adjusted odds ratio of total CVD from a restricted cubic spline logistic regression model with knots at the 5th, 35th, 65th, and 95th percentiles. Data are ORs (solid line) and 95% CIs (dashed lines) from multivariate logistic regression analysis with restricted cubic splines. Abbreviations: WWI, weight-adjusted-waist index; CVD, cardiovascular disease; OR, odds ratio; CI, confidence interval
Association between WWI and specific CVDs
We further analyzed the associations between WWI and the prevalence of five specific CVDs (CHF, CHD, angina, heart attack, and stroke) (Additional File Table 2). After adjusting for all covariates (Model 3), the fifth quintile of WWI was positively associated with the increased prevalence of CHF (quintile 5: OR = 2.67, 95% CI: 1.12–6.33, P = 0.026), angina (quintile 5: OR = 3.16, 95% CI: 1.05–9.54, P = 0.041) and heart attack (quintile 5: OR = 13.68, 95% CI: 4.93–38.03, P < 0.001), and the fourth quintile of WWI was associated with the increased prevalence of CHD (quintile 4: OR = 2.57, 95% CI: 1.06–6.27, P = 0.037).
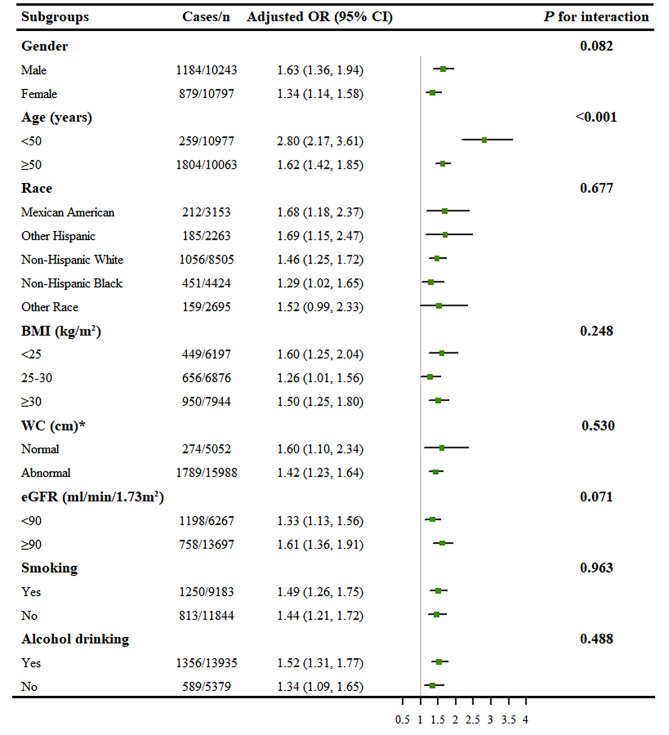
Subgroup analysis
We performed a further stratified analysis to assess the effect of WWI on CVD in various subgroups (Fig. 2). None of the variables, including gender (female or male), race (Mexican American, other Hispanic, non-Hispanic white, non-Hispanic black and other race), BMI (< 25 or 25–30 or ≥ 30 kg/m2), WC (normal or abnormal), eGFR (< 90 or ≥ 90 ml/min/1.73m2), smoking (yes or no), and alcohol drinking (yes or no) significantly modified the association between WWI and CVD (all P for interaction > 0.05). Nevertheless, there was a significant interaction between WWI and age (< 50 or ≥ 50 years) on CVD. A stronger positive association between WWI and CVD was found in participants younger than 50 years of age (OR = 2.80, 95% CI: 2.17–3.61) compared with their counterparts (OR = 1.62; 95% CI: 1.42–1.85) (P for interaction < 0.001).
Fig. 2.
Subgroup analyses of the association between WWI and CVD in US adults. All presented covariates were adjusted (as Model 3) except the corresponding stratification variable. * Normal: WC < 94 cm in male and WC < 80 cm in female; abnormal: WC ≥ 94 cm in male and WC ≥ 80 cm in female. Abbreviations: WWI, weight-adjusted-waist index; CVD, cardiovascular disease; US, the United States; BMI, body mass index; WC, waist circumference; eGFR, estimated glomerular filtration rate; OR, odds ratio; CI, confidence interval
ROC curves between WWI and traditional obesity indices
The three traditional obesity indices (BMI, WC, and WHtR) were significantly correlated with one another, with Pearson correlation coefficients that ranged from r = 0.908 to 0.930; however, there was a fair correlation between WWI and those indices (> 0.5 r < 0.8) (Additional File Table 3). The ROC curves of the different indices for CVD are shown in Table 3. For both males and females, WWI had the highest area under the curve (AUC) of 0.736 (95% CI: 0.722–0.751, P < 0.05) and 0.677 (95% CI: 0.659–0.694, P < 0.05), with a cut-off value of 11.11 cm/√kg and 11.47 cm/√kg, respectively.
Table 3.
Comparison of the ability of different obesity-related indices to predict CVD.
| Test | AUC (95% CI) | Cut-off | Specificity | Sensitivity | Youden index | P value |
|---|---|---|---|---|---|---|
| Males | ||||||
| BMI | 0.567 (0.549, 0.584) | 27.86 | 0.523 | 0.579 | 0.102 | < 0.001 |
| WC | 0.638 (0.622, 0.653) | 97.15 | 0.463 | 0.752 | 0.215 | < 0.001 |
| WHtR | 0.659 (0.643, 0.674) | 0.58 | 0.542 | 0.705 | 0.247 | < 0.001 |
| WWI | 0.736 (0.722, 0.751) | 11.11 | 0.654 | 0.709 | 0.363 | < 0.001 |
| Females | ||||||
| BMI | 0.569 (0.550, 0.589) | 29.62 | 0.579 | 0.533 | 0.112 | < 0.001 |
| WC | 0.612 (0.593, 0.631) | 104.45 | 0.699 | 0.464 | 0.163 | < 0.001 |
| WHtR | 0.629 (0.610, 0.647) | 0.59 | 0.504 | 0.694 | 0.198 | < 0.001 |
| WWI | 0.677 (0.659, 0.694) | 11.47 | 0.619 | 0.639 | 0.258 | < 0.001 |
Abbreviations: WWI, weight-adjusted-waist index; BMI, body mass index; WHtR, waist-to-height ratio; WC, waist circumference; AUC, area under the curve; CI, confidence interval
Discussion
To our knowledge, this cross-sectional study was the first to demonstrate the relationship between WWI and CVD in a nationally representative sample of the US adult population. The results indicated that WWI was significantly associated with an increased risk of CVD, presenting a nearly linear dose-response relationship. Furthermore, the subgroup analyses showed that a stronger association between WWI and CVD was detected in participants younger than 50 years of age (P for interaction < 0.001). Besides, the ROC curve showed that WWI had better identification performance for CVD than BMI, WC, and WHtR.
Obesity, a condition of excessive body fat accumulation caused by long-term energy intake exceeding energy expenditure, is a well-established risk factor for CVD and mortality in adult populations [5]. Hence, measurement of body fat has been a critical issue in clinical practice for assessing obesity and then identifying individuals at risk of CVD. Although cumulative evidence shows that those traditional obesity-related indices (such as BMI, WC, and waist-to-hip ratio [WHR]) are associated with CVD risk, the obesity paradox still exists, partly because the significant correlations between various indicators hamper the identification of biologically driven risks for diseases (as shown in Table S3) [7, 22, 23]. Another explanation for the paradox may be that BMI cannot distinguish between an elevated weight caused by high levels of lean vs. fat body mass. In this context, nontraditional obesity indicators (such as body shape index [ABSI], cardiometabolic index [CMI], and visceral adiposity index [VAI]) were also employed to assess the exact relationship between them and find positive associations [24–26]. For example, a prospective study of 3,042 participants found that VAI was significantly associated with the 10-year CVD incidence (HR = 1.05, 95% CI: 1.01–1.10) [26]. Some scholars also put forward “metabolic health” based on WHR, systolic BP, and self-reported diabetes and found it to be clearly associated with CVD and total mortality, regardless of BMI [27]. However, those parameters are complex to calculate or susceptible to subjectivity, making it inconvenient to conduct routine examinations in the general population.
WWI is a simple anthropometric indicator derivated according to the formula [In (WC) = β0 + β1 In (weight) + ε], which standardizes WC for weight by the least squared regression of the logarithm-transformed WC on the logarithm-transformed weight [15]. Hence, WWI may combine the advantages of WC while weakening the correlation with BMI, enabling it to evaluate both fat mass and muscle mass. Indeed, a recent study involving 602 participants showed that WWI was positively correlated with total and abdominal fat measures but negatively correlated with appendicular skeletal muscle mass in older adults [28]. Moreover, WWI was significantly higher in the metabolically unhealthy group than in the metabolically healthy group, even with similar levels of obesity [29]. Thus, WWI can reflect “true obesity” that is metabolically unhealthy. Several studies have demonstrated that WWI is positively correlated with CVD mortality in East Asian populations [15–17]. Our study further verified the association between WWI and CVD in the US adult population. Furthermore, the WWI showed better identification performance for CVD than BMI, WC, and WHtR. These findings suggest that WWI may be a superior indicator of obesity, which is not limited to East Asians but generally applicable to diverse populations.
Due to the substantial differences in body composition by race, anthropometric indicators such as BMI have different cut-points for identifying obesity according to race [30]. For instance, the risk of diabetes in Chinese populations with a BMI of 26.9 kg/m² was the same as that of White populations with a BMI of 30 kg/m² [31]. Nevertheless, in the case of WWI, there were no statistically significant differences in the mean and distribution between Whites, Asians, and African Americans, supporting our current findings [32]. This may be related to the fact that WWI measures the ratio of fat and muscle mass rather than the absolute fat amount. Additionally, the Korean Frailty and Aging Cohort study showed that WWI was strongly associated with sarcopenic obesity, which is defined as the presence of high fat mass and low muscle mass combined with low physical function [33]. Therefore, elevated WWI reflects a state of excessive body fat accumulation and increased muscle mass loss. The muscle-fat imbalance results in dysregulation of adipocytokine release, inflammatory responses, endothelial dysfunction, and declined physical function, ultimately leading to the development of CVD [34–36].
The subgroup analysis demonstrated that the WWI-CVD association was more significant in participants younger than 50 years of age (P for interaction < 0.001). Similar to our findings, a study by Cai showed that the association between WWI and all-cause mortality was not significant at age ≥ 75 years [17]. Moreover, in the Rural Chinese Cohort Study, Li et al. found an association between WWI and hypertension in people under 60 years of age, but it disappeared in older adults (age ≥ 60 years) [37]. These results might be attributed to the different body fat distribution between older and younger individuals [38]. Moreover, subjects with obesity (BMI ≥ 30 kg/m2) had a higher risk for CVD than normal-weight individuals, indicating the obese population could be more vulnerable to the increase in WWI. Taken together, the risk of CVD assessed by WWI may be more advantageous for younger obese people. Besides, we found that WWI prediction ability for CVD in the Other Race subgroup was least validated, despite the fact that this measure was developed using an East Asian population. Due to the decreased sample size after stratification and the inclusion of a multi-racial population, which may lead to some potential biases, the results need to be verified in the future with larger, more specific populations.
Our study has important implications for clinical practice. First, we found a positive association between WWI and CVD in populations outside East Asia, suggesting that WWI could be a universal health index that applies to various races or ethnic groups. Besides, WWI is a better measure of obesity than BMI, WC, and WHtR, and its association with CVD is also stronger than those traditional indices. Hence, for people with high WWI levels, early assessment of target organ damage and timely intervention may reduce the risk of CVD and improve outcomes. Moreover, due to its simple calculation and economic nature, WWI can be used by medical and health institutions at all levels, especially in areas where medical resources are limited.
This study has several strengths. First of all, we used a national representative sample of the general adult population of the US from NHANES, which applied rigorous study protocols and quality controls. Secondly, we adjusted for most confounding covariates to ensure that our findings were reliable. Lastly, we used MI to maximize statistical power and minimize bias that might occur if covariates with missing data were excluded from data analyses. However, the limitations should also be noted. Due to the cross-sectional nature of NHANES, we could not obtain a causal relationship between WWI and CVD. Therefore, further longitudinal studies are needed to verify these findings. Moreover, although we have adjusted for many confounding covariates, we could not rule out any potential residual confounding, such as genetic factors and drug use. Besides, measurement errors in WC (such as the location of the waist area and individual variance in positioning the measuring tape) and CVD determined from participants’ self-reported data may lead to some potential bias. However, previous studies have demonstrated that NHANES self-reported outcomes are a valid method for determining prevalence [39]. Although WWI is a better indicator of obesity, implementing it into the current US health care system remains a challenge.
Conclusions
Our study demonstrated that high levels of WWI were significantly associated with an increased risk of CVD in US adults, particularly in people under 50 years of age. These findings indicate that WWI may be an intervention indicator to reduce the risk of CVD in the general adult population. However, further longitudinal studies are still needed to clarify the precise causality of this relationship.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Additional File 1: Supplementary Appendix
Acknowledgements
We thank all the National Health and Nutrition Examination Survey (NHANES) participants and staff for their valuable efforts and contributions.
Abbreviations
- NHANES
National Health and Nutrition Examinations Survey
- CVD
Cardiovascular disease
- BMI
Body mass index
- WC
Waist circumference
- WHtR
Waist-to-height ratio
- WWI
Weight-adjusted-waist index
- NCHS
National Center for Health Statistics
- CDC
Centers for Disease Control and Prevention
- BP
Blood pressure
- HbA1c
Hemoglobin A1c
- TBIL
Total bilirubin
- LDL-C
Low-density lipoprotein cholesterol
- HDL-C
High-density lipoprotein cholesterol
- TC
Total cholesterol
- TG
Triglycerides
- Scr
Serum creatinine
- SUA
Serum uric acid
- UACR
Urinary albumin/creatinine ratio
- MEC
Mobile Examination Centre
- eGFR
Estimated glomerular filtration rate
- CHF
Congestive heart failure
- CHD
Coronary heart disease
- SD
Standard deviation
- OR
Odds ratios
- CI
Confidence intervals
- ROC
Receiver operating characteristic
- MI
Multiple imputation
- AUC
Area under the curve
Author contributions
FX and YQW conceived and designed the study. HYF, KL, and ML contributed to data collection and statistical analysis. HYF and FX drafted the manuscript. YQW had primary responsibility for the final content of the manuscript. All authors interpreted the results, and reviewed and approved the manuscript.
Funding
This work was supported by the National Key Research and Development Plan of China (No. 20212BBG71004), the National Natural Science Foundation of China (No. 82160085), the Jiangxi Provincial Department of Science and Technology Foundation (No. 20181BCB24013), and the Natural science funding (No. 20202BAB206005).
Data Availability
The datasets that support the findings of this study are available from the National Center for Health Statistics (https://www.cdc.gov/nchs/nhanes/index.htm) or from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
All methods were carried out in accordance with the Declaration of Helsinki. The Ethics Review Board of the National Center for Health Statistics approved all NHANES protocols and written informed consents were obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Haiyang Fang and Feng Xie contribute equally to this work.
References
- 1.Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6–10. doi: 10.1016/j.metabol.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 2.NCD Risk Factor Collaboration NCD-RisC Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387:1377–96. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hales CM, Carroll MD, Fryar CD et al. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief 2017: 1–8. [PubMed]
- 4.van Dis I, Kromhout D, Geleijnse JM, et al. Body mass index and waist circumference predict both 10-year nonfatal and fatal cardiovascular disease risk: study conducted in 20,000 dutch men and women aged 20–65 years. Eur J Cardiovasc Prev Rehabil. 2009;16:729–34. doi: 10.1097/HJR.0b013e328331dfc0. [DOI] [PubMed] [Google Scholar]
- 5.Khan SS, Ning H, Wilkins JT, et al. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol. 2018;3:280–7. doi: 10.1001/jamacardio.2018.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang M, Zhao Y, Wang G, et al. Body mass index and waist circumference combined predicts obesity-related hypertension better than either alone in a rural chinese population. Sci Rep. 2016;6:31935. doi: 10.1038/srep31935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell DJ, Gong FF, Jelinek MV, et al. Threshold body mass index and sex-specific waist circumference for increased risk of heart failure with preserved ejection fraction. Eur J Prev Cardiol. 2019;26:1594–602. doi: 10.1177/2047487319851298. [DOI] [PubMed] [Google Scholar]
- 8.Flegal KM, Kit BK, Orpana H, et al. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hainer V, Aldhoon-Hainerová I. Obesity paradox does exist. Diabetes Care. 2013;36(Suppl 2):276–81. doi: 10.2337/dcS13-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uretsky S, Messerli FH, Bangalore S, et al. Obesity paradox in patients with hypertension and coronary artery disease. Am J Med. 2007;120:863–70. doi: 10.1016/j.amjmed.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Romero-Corral A, Somers VK, Sierra-Johnson J, et al. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes (Lond) 2008;32:959–66. doi: 10.1038/ijo.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antonopoulos AS, Oikonomou EK, Antoniades C, et al. From the BMI paradox to the obesity paradox: the obesity-mortality association in coronary heart disease. Obes Rev. 2016;17:989–1000. doi: 10.1111/obr.12440. [DOI] [PubMed] [Google Scholar]
- 13.Pasdar Y, Moradi S, Moludi J, et al. Waist-to-height ratio is a better discriminator of cardiovascular disease than other anthropometric indicators in kurdish adults. Sci Rep. 2020;10:16228. doi: 10.1038/s41598-020-73224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kammar-García A, Hernández-Hernández ME, López-Moreno P, et al. Relation of body composition indexes to cardiovascular disease risk factors in young adults. Semergen. 2019;45:147–55. doi: 10.1016/j.semerg.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Park Y, Kim NH, Kwon TY, et al. A novel adiposity index as an integrated predictor of cardiometabolic disease morbidity and mortality. Sci Rep. 2018;8:16753. doi: 10.1038/s41598-018-35073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding C, Shi Y, Li J, et al. Association of weight-adjusted-waist index with all-cause and cardiovascular mortality in China: a prospective cohort study. Nutr Metab Cardiovasc Dis. 2022;32:1210–7. doi: 10.1016/j.numecd.2022.01.033. [DOI] [PubMed] [Google Scholar]
- 17.Cai S, Zhou L, Zhang Y, et al. Association of the weight-adjusted-waist index with risk of all-cause mortality: a 10-year follow-up study. Front Nutr. 2022;9:894686. doi: 10.3389/fnut.2022.894686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zipf G, Chiappa M, Porter KS, et al. National health and nutrition examination survey: plan and operations, 1999–2010. Vital Health Stat. 2013;1:1–37. [PubMed] [Google Scholar]
- 19.Inker LA, Eneanya ND, Coresh J, et al. New Creatinine- and cystatin C-Based equations to Estimate GFR without Race. N Engl J Med. 2021;385:1737–49. doi: 10.1056/NEJMoa2102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu C, Weng Z, Zhang L, et al. HDL cholesterol: a potential mediator of the association between urinary cadmium concentration and cardiovascular disease risk. Ecotoxicol Environ Saf. 2021;208:111433. doi: 10.1016/j.ecoenv.2020.111433. [DOI] [PubMed] [Google Scholar]
- 21.Johnson CL, Paulose-Ram R, Ogden CL, et al. National health and nutrition examination survey: analytic guidelines, 1999–2010. Vital Health Stat. 2013;2:1–24. [PubMed] [Google Scholar]
- 22.Kim NH, Lee J, Kim TJ, et al. Body Mass Index and Mortality in the General Population and in subjects with chronic disease in Korea: a Nationwide Cohort Study (2002–2010) PLoS ONE. 2015;10:e0139924. doi: 10.1371/journal.pone.0139924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fenger-Grøn M, Overvad K, Tjønneland A, et al. Lean body Mass is the predominant anthropometric risk factor for Atrial Fibrillation. J Am Coll Cardiol. 2017;69:2488–97. doi: 10.1016/j.jacc.2017.03.558. [DOI] [PubMed] [Google Scholar]
- 24.Zhou W, Zhu L, Yu Y et al. A body shape index is positively associated with all-cause and cardiovascular disease mortality in the chinese population with normal weight: a prospective cohort study. Nutr Metab Cardiovasc Dis. 2023. [DOI] [PubMed]
- 25.Cai X, Hu J, Wen W, et al. Associations of the Cardiometabolic Index with the risk of Cardiovascular Disease in patients with hypertension and obstructive sleep apnea: results of a Longitudinal Cohort Study. Oxid Med Cell Longev. 2022;2022:4914791. doi: 10.1155/2022/4914791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kouli GM, Panagiotakos DB, Kyrou I, et al. Visceral adiposity index and 10-year cardiovascular disease incidence: the ATTICA study. Nutr Metab Cardiovasc Dis. 2017;27:881–9. doi: 10.1016/j.numecd.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 27.Zembic A, Eckel N, Stefan N, et al. An empirically derived definition of metabolically healthy obesity based on risk of Cardiovascular and total mortality. JAMA Netw Open. 2021;4:e218505. doi: 10.1001/jamanetworkopen.2021.8505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim NH, Park Y, Kim NH, et al. Weight-adjusted waist index reflects fat and muscle mass in the opposite direction in older adults. Age Ageing. 2021;50:780–6. doi: 10.1093/ageing/afaa208. [DOI] [PubMed] [Google Scholar]
- 29.Abolnezhadian F, Hosseini SA, Alipour M, et al. Association metabolic obesity phenotypes with Cardiometabolic Index, Atherogenic Index of plasma and Novel Anthropometric Indices: a link of FTO-rs9939609 polymorphism. Vasc Health Risk Manag. 2020;16:249–56. doi: 10.2147/VHRM.S251927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev. 2002;3:141–6. doi: 10.1046/j.1467-789X.2002.00065.x. [DOI] [PubMed] [Google Scholar]
- 31.Caleyachetty R, Barber TM, Mohammed NI, et al. Ethnicity-specific BMI cutoffs for obesity based on type 2 diabetes risk in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2021;9:419–26. doi: 10.1016/S2213-8587(21)00088-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JY, Choi J, Vella CA, et al. Associations between Weight-Adjusted Waist Index and Abdominal Fat and muscle Mass: multi-ethnic study of atherosclerosis. Diabetes Metab J. 2022;46:747–55. doi: 10.4093/dmj.2021.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JE, Choi J, Kim M, et al. Assessment of existing anthropometric indices for screening sarcopenic obesity in older adults. Br J Nutr. 2023;129:875–87. doi: 10.1017/S0007114522001817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamjane N, Benyahya F, Nourouti NG, et al. Cardiovascular diseases and metabolic abnormalities associated with obesity: what is the role of inflammatory responses? A systematic review. Microvasc Res. 2020;131:104023. doi: 10.1016/j.mvr.2020.104023. [DOI] [PubMed] [Google Scholar]
- 35.Evans K, Abdelhafiz D, Abdelhafiz AH. Sarcopenic obesity as a determinant of cardiovascular disease risk in older people: a systematic review. Postgrad Med. 2021;133:831–42. doi: 10.1080/00325481.2021.1942934. [DOI] [PubMed] [Google Scholar]
- 36.Singhal A. Endothelial dysfunction: role in obesity-related disorders and the early origins of CVD. Proc Nutr Soc. 2005;64:15–22. doi: 10.1079/PNS2004404. [DOI] [PubMed] [Google Scholar]
- 37.Li Q, Qie R, Qin P, et al. Association of weight-adjusted-waist index with incident hypertension: the rural chinese cohort study. Nutr Metab Cardiovasc Dis. 2020;30:1732–41. doi: 10.1016/j.numecd.2020.05.033. [DOI] [PubMed] [Google Scholar]
- 38.Szulc P, Duboeuf F, Chapurlat R. Age-related changes in fat mass and distribution in men-the cross-sectional STRAMBO study. J Clin Densitom. 2017;20:472–9. doi: 10.1016/j.jocd.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Lopez-Jimenez F, Batsis JA, Roger VL, et al. Trends in 10-year predicted risk of cardiovascular disease in the United States, 1976 to 2004. Circ Cardiovasc Qual Outcomes. 2019;2:443–50. doi: 10.1161/CIRCOUTCOMES.108.847202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional File 1: Supplementary Appendix
Data Availability Statement
The datasets that support the findings of this study are available from the National Center for Health Statistics (https://www.cdc.gov/nchs/nhanes/index.htm) or from the corresponding author upon reasonable request.