Abstract
Introduction:
A traceback genetic testing program for ovarian cancer has the potential to identify individuals with hereditary breast and ovarian cancer and their relatives. Successful implementation depends on understanding and addressing the experiences, barriers, and preferences of the people served.
Methods:
We conducted a remote, human-centered design research study of people with ovarian, fallopian tube, or peritoneal cancer (probands) and people with a family history of ovarian cancer (relatives) at three integrated health systems between May and September 2021. Participants completed activities to elicit their preferences about ovarian cancer genetic testing messaging, and to design their ideal experience receiving an invitation to participate in genetic testing. Interview data were analyzed using a rapid thematic analysis approach.
Results:
We interviewed 70 participants and identified five preferred experiences for a traceback program. Participants strongly prefer discussing genetic testing with their doctor but are comfortable discussing with other clinicians. The most highly preferred experience for both probands and relatives was to discuss with a knowledgeable clinician who can answer questions, followed by directed (sent directly to specific people) or passive (shared in a public area) communication. Repeated contact was acceptable for reminders.
Discussion/Conclusion:
Participants were open to receiving information about traceback genetic testing and recognized its value. Participants preferred discussing genetic testing with a trusted clinician. Directed communication was preferable to passive communication. Other valued information included how genetic tests help their family and the cost of genetic testing. These findings are informing traceback cascade genetic testing programs at all three sites.
Keywords: Ovarian cancer, genetic testing, human-centered design, traceback, cascade testing
Introduction
Ovarian cancer is the deadliest gynecologic cancer and the fifth leading cause of cancer death in the US for women[1,2]. Genetic testing of everyone with ovarian cancer improves detection of BRCA and other high-risk genetic variants and is the current standard of care for people with newly diagnosed ovarian cancer[3]. However, despite guidelines recommending genetic testing for everyone with ovarian cancer and their at-risk relatives, fewer than one-quarter are tested[4]. A “traceback” cascade testing approach, where people with ovarian cancer and their relatives are retrospectively contacted and offered testing, is a possible solution[5]. In a traceback program, individuals with a history of ovarian cancer who did not receive genetic testing at the time of diagnosis are identified using cancer registries and electronic health records (EHRs). Once identified, the health system contacts them and invites them to receive genetic testing. When genetic testing identifies a concerning variant with implications for the person’s biologic family members, the health system works with the person to identify and contact at-risk family members and invite them to receive genetic testing for the same variant. However, the feasibility and communication strategies involved in this type of outreach are not well understood. Traceback programs are new for U.S. settings, and understanding the needs and preferences of patients and family members is critical for successful program implementation.
The objective of this study was to identify language and communication preferences for traceback programs. We used human-centered design research methods to engage a diverse sample of people with a personal history of ovarian, fallopian tube, or peritoneal cancer, and people with family history of these cancers, to determine preferences for language, communication, and processes to be used in a traceback program. We co-designed a patient-centered intervention with probands and relatives as part of an ongoing project to determine the acceptability, feasibility, and effectiveness of a traceback cascade screening program in three integrated health systems[6].
Materials and Methods
Design and setting
We conducted a qualitative, human-centered design study. Human-centered design uses principles and methods from other disciplines, including design, anthropology, sociology, and psychology, to deeply understand the person (such as a patient or family member) for whom a resource or service is being designed. Human-centered design methodologies have been used to design and implement innovative solutions to complex problems across all industries, including health care[7–11]. The goal is to pair what is desired by the person with what is technically feasible and economically viable[12,13] often by conducting qualitative interviews and observations to understand their experiences, preferences, barriers, and facilitators related to the problem and possible solutions[14]. The human-centered design researcher facilitates activities to engage the person in the process of designing an ideal experience; thus eliciting a foundational understanding of the problem the person is experiencing and how to solve it[15].
The setting for this study was three integrated health systems: Geisinger Health System, Kaiser-Permanente Mid-Atlantic States (KPMAS), and Kaiser-Permanente Washington (KPWA). Study teams from the three systems’ research institutes are collaborating on an NIH-funded study examining the feasibility of traceback genetic testing in families with a history of ovarian cancer. All study activities were approved by the Geisinger Institutional Review Board.
Participant selection and sampling
The populations of interest were: (1) individuals receiving care at study sites who have a prior history of ovarian, fallopian tube, or peritoneal cancer (probands); (2) individuals without a personal prior history but with a family history of these cancers (relatives). Relatives for this study did not need to be biologically related to participating probands.
Eligible probands and relatives were identified through EHR data using distributed query code through the virtual data warehouse (VDW)[16]. Included probands were those who received a diagnosis of ovarian, peritoneal, or fallopian tube cancer between 1980 and 2020, and who were living and receiving care as members at one of the study sites. Included relatives were living adults with EHR documentation of family history of ovarian, peritoneal, or fallopian tube cancer. Hospice patients were excluded for both probands and relatives.
Participants were a random sample of individuals who had and had not received genetic testing. KPWA and Geisinger used a stratified sampling approach for probands based on time from diagnosis (50% less than 5 years from diagnosis; 50% 5 or more years from diagnosis). Geisinger also included a stratified sampling approach for relatives based on the vital status of their relative who has or had ovarian, peritoneal, or fallopian tube cancer, aiming to recruit both relatives of living and deceased persons who received an ovarian, peritoneal, or fallopian tube cancer diagnosis. KPMAS only contacted individuals who had received genetic testing and used race/ethnicity-based sampling with no time from diagnosis criteria, recruiting Black patients first.
Eligible participants were contacted by mail, inviting them to participate in an interview, followed by telephone contact to schedule an interview. One study site (KPMAS) also conducted social media-based outreach to members, to invite probands and relatives to self-identify as potentially eligible to participate.
Data collection
Interviews were conducted by trained, masters-prepared (PB, AD, LS [MPH candidate]) or PhD-prepared (KR, AB) study team members with previous experience conducting qualitative or human-centered design interviews, as well as by certified genetic counselors (ZS, RS) whose clinical practice involves recommending genetic testing for hereditary breast and ovarian cancer risk. Notetakers were trained study team members with at least bachelors’ degrees (TK, IL, LS). All interviewers had at least 2 years of prior qualitative research experience, including interviewing. Interviewers ages ranged from mid-20s to mid-60s and were either White race or multiracial (Asian and White). Interviewers reflected during the training on how their training and personal experiences could influence their conduct of interviews and participant experience; for example, no Black participants were able to have race-concordant interviews. All interviews occurred between May and September 2021. Interviews were conducted via phone or video call on Microsoft Teams, chosen at the discretion of the participant. All interviews were audiorecorded and transcribed verbatim. Interviews lasted approximately 90 minutes. At least two study team members participated in each interview, with one as the main interviewer and one designated as note-taker.
Data collection instruments.
We developed two semi-structured interview guides (Supplementary Material: Probands Interview Guide and Relatives Interview Guide) one for probands and one for relatives. Interview topics included experience(s) with ovarian cancer, experience(s) with genetic testing in general, and experience(s) with testing for genetic markers associated with cancer. The interview guide also included a brief demographic survey. Before proceeding with interviews, we requested feedback from the KPMAS Community Advisory Board (CAB)[17], reviewed proposed content with a literacy consultant, and pre-tested the final interview guide with community members identified through the cancer genetics clinic at Geisinger.
Design persona and artifacts.
Personas and artifacts are tools commonly used in participatory, human-centered design methodology for rapidly creating a shared sense of the intended user, to understand their perspectives, and to co-design an experience or process with them[14]. We created two personas: Sam, an individual with ovarian cancer, and Pat, a relative of an individual with ovarian cancer (Supplementary Material: Persona Artifacts). Both Sam and Pat are considering genetic testing for ovarian cancer risk. Both personas were written to be gender ambiguous to represent transgender and non-binary people in addition to cis-female patients.
Because we could not conduct interviews in person due to the COVID-19 pandemic, we developed a series of artifacts to be used interactively during the remote interviews[18] (Supplementary Materials: Persona Artifacts). The artifacts included (1): A list of information statements about genetic testing for hereditary ovarian cancer (“Ovarian Cancer Statements”), designed to elicit preferences on messages to be used in outreach for traceback testing; and (2) Storyboard panels depicting the persona receiving communication about genetic testing for hereditary ovarian cancer in a variety of ways (“Modes”), designed to generate the preferred modes of contact.
Interview procedures
Interviewers were trained in how to conduct design-focused interviews by a human-centered design expert (KR). The interviewers also observed KR pilot the interview guide and associated artifacts with two pilot participants (one proband, and one relative). The interviewers consulted the study team to address interview issues, questions, and clarifications during the data collection period.
Before each scheduled interview, the study team mailed or emailed a packet to the participant that contained all artifacts to be referred to in the interview. Each participant’s packet included a list of statement artifacts and a set of storyboard panel artifacts. Statements were randomized to reduce order effect bias, where placement in a list of responses influences which responses the participant chooses[19]. Each storyboard panel was printed on an individual sheet of paper. The panels were manually shuffled prior to mailing to reduce order effect.
After asking the initial set of background questions, we oriented participants to the persona and a “Choose Your Own Adventure” activity[20] explaining we were asking them to design their own preferred experience from the perspective of Sam or Pat receiving an invitation to participate in genetic testing. We asked participants to identify up to five statements which were important information that must be included, and up to five statements which should not be included. We guided participants to the storyboard panels, then asked them to identify preferred modes of receiving information about ovarian cancer genetic testing. As a closing activity, the interviewer conducted member-checking by restating the participants’ preferred total experience and their reasons for choosing the experience and asking for feedback.
During the interview, the designated note-taker captured the participants’ responses to the activities on the online whiteboard [21] to create a unique whiteboard for each participant containing their preferred messages and storyboard panels. Participants could see the whiteboard if they opted to participate via remote video call, but most participants chose to participate via telephone only. Interviews were recorded and transcribed by a professional transcription service.
Analysis
Interviewers completed field notes within 24 hours of each interview, including context, highlights of the interview, the participant story, major findings, and notable quotes; and wrote episodic summaries based on a template derived from the interview guide[22]. Study data were collected and managed using REDCap electronic data capture tools hosted at Kaiser-Permanente Washington[23,24]. REDCap (Research Electronic Data Capture) is a secure, web-based software platform designed to support data capture for research studies.
We used content analysis, affinity mapping [14,25] and rapid qualitative data analysis methods[26] We summarized frequencies of the participant-preferred messages and modes at each study site. We analyzed ideal experience storyboards using affinity diagramming, a collaborative activity to organize related or similar storyboards into distinct, mutually exclusive clusters. The study team participated in several virtual affinity diagramming workshops led by KR, where we grouped subsets of the storyboards by various categories. The affinity diagramming groups from the workshop were the foundation for iterative affinity diagramming exercises lead by KR with input from AKR, CJ, and NBH. We synthesized participant preferences into a series of experiences, which we defined as the combination of multiple modes, with common themes in the type of mode (in person, directed, or passive) and the timing of the communication (outreach before, communication during, or follow-up after an appointment).
Emergent themes around preferred messages and modes were analyzed by NBH, PB, CB, AD, TK, ZS and IL using a rapid, thematic approach[27]. Interview transcripts were coded using a template analysis approach, entering summaries and exemplar quotes into the study database. We then prepared a series of coding memos to summarize data across sites, including emergent themes not identified in the template. To triangulate the findings, REDCap data were exported to Excel, then organized as positive, negative, or neutral, and quantified. These categorized data were then exported, labeled with subcategory tags facilitated by the software program, and compared with the previously written summaries. We further synthesized the findings into a set of design requirements for traceback programs.
Results
We invited 655 people to participate in the study. We conducted 70 semi-structured phone and/or video interviews with ovarian, peritoneal, or fallopian tube cancer patients and survivors (probands) and people who had a family history of ovarian, peritoneal, or fallopian tube cancer (relatives), between May and September 2021 (Figure 1). Seventy participants is a substantially larger sample size than the typical number necessary to reach saturation in qualitative research[28]; however, it was essential to engage a sufficient number of participants at each study site to inform culturally-appropriate language and communication strategies specific to the population and region each healthcare system served. Saturation was reached at each site when the interviewers and site leads determined they were no longer hearing new concepts from the interviews.
Figure 1:
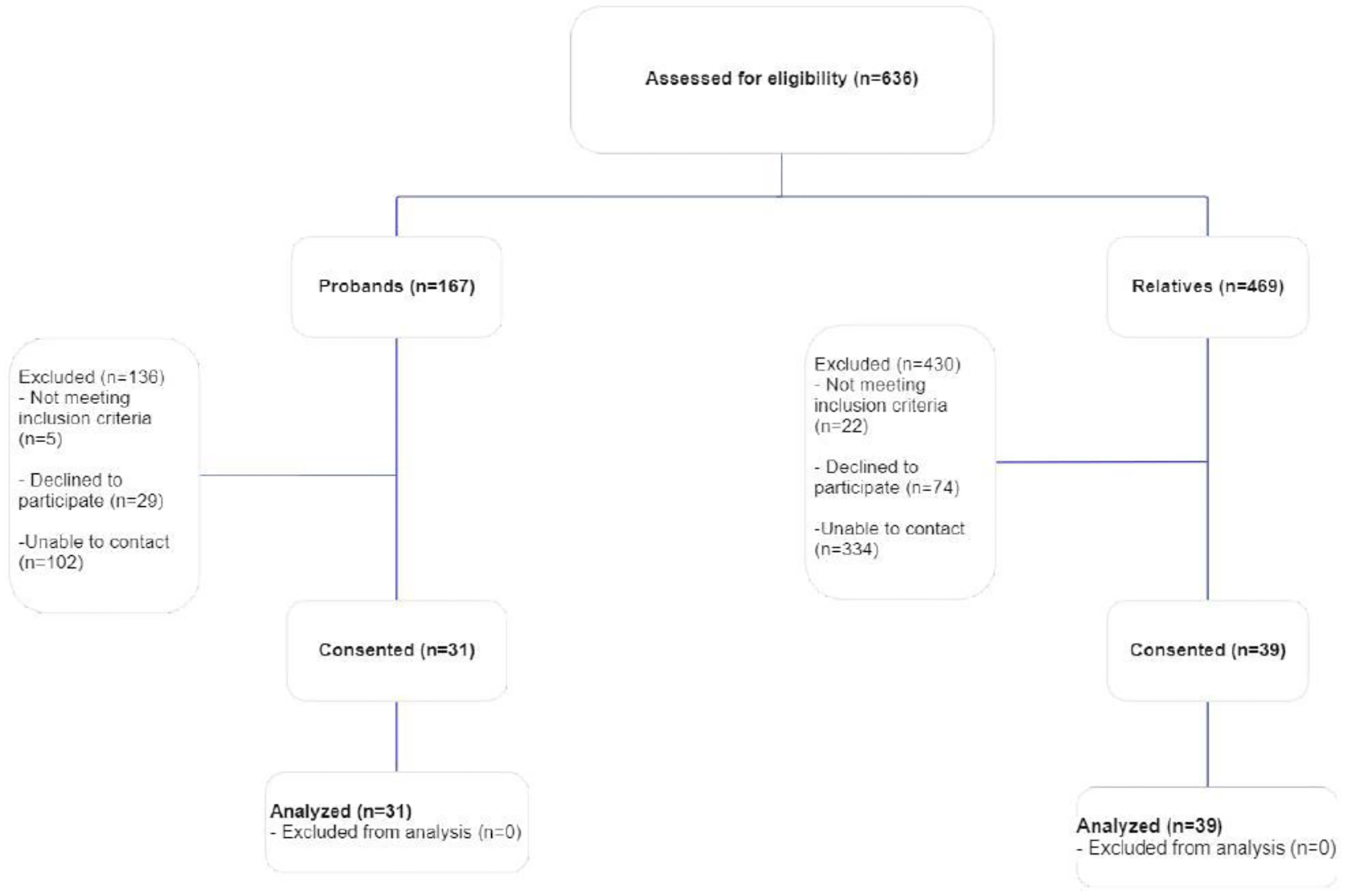
Consort diagram of recruitment of study participants
Table 1 describes the demographics of the participants. Briefly, participants included 31 people with ovarian, peritoneal, or fallopian tube cancer (10 Geisinger; 11 KPMAS; 10 KPWA), and 39 people who had a family history of ovarian, peritoneal, or fallopian tube cancer (21 Geisinger; 8 KPMAS; 10 KPWA). The mean age of all participants was above 60 years old, and most (56%) participants received college- or post-graduate level of education. The majority (78%) of participants had commercial and/or private insurance, and 34% of participants reporting working for pay at the time of the interview.
Table 1:
Sample characteristics
| All | Probands | Relatives | ||||
|---|---|---|---|---|---|---|
| n=70 | % | n=31 | % | n=39 | % | |
| Geisinger | 31 | 10 | 21 | |||
| KPMAS | 19 | 11 | 8 | |||
| KPWA | 20 | 10 | 10 | |||
| Assigned female at birth | 68 | 97% | 31 | 100% | 37 | 95% |
| Assigned male at birth | 2 | 3% | 2 | 5% | ||
| Mean age (min, max) | 61.8 (25, 89) | 63.7 (34, 89) | 58.2 (25, 85) | |||
| Education | ||||||
| High school or less | 14 | 20% | 5 | 16% | 9 | 23% |
| Some college or trade, technical, or vocational school | 17 | 24% | 5 | 16% | 12 | 31% |
| College graduate | 21 | 30% | 10 | 32% | 11 | 28% |
| Postgraduate | 18 | 26% | 11 | 36% | 7 | 18% |
| Health insurance | ||||||
| Medicare | 25 | 36% | 14 | 45% | 11 | 28% |
| Commercial/private | 54 | 78% | 24 | 77% | 30 | 77% |
| Other (Medicaid and/or Military/Tricare) | 3 | 4% | 2 | 6% | 1 | 3% |
| No insurance | 2 | 3% | 1 | 3% | 1 | 3% |
| Prefer not to answer | 1 | 1% | 1 | 3% | 0 | 0% |
| Hispanic ethnicity | 1 | 1% | 1 | 3% | ||
| Race | ||||||
| Black or African American | 10 | 14% | 3 | 10% | 7 | 18% |
| White | 54 | 77% | 25 | 81% | 29 | 74% |
| Asian | 5 | 7% | 3 | 10% | 2 | 5% |
| More than one race | 1 | 1% | 1 | 3% | ||
| Marital status | ||||||
| Single/never married | 14 | 20% | 7 | 23% | 7 | 23% |
| Married | 38 | 54% | 19 | 61% | 19 | 61% |
| Widowed / separated / divorced | 11 | 16% | 5 | 16% | 6 | 19% |
| Currently working for pay=yes | 24 | 34% | 10 | 32% | 14 | 36% |
| Household income (combined, pre-tax) | ||||||
| $50,000 or less | 16 | 23% | 6 | 20% | 13 | 33% |
| $50,000-$150,000 | 28 | 40% | 15 | 48% | 13 | 34% |
| More than $150,000 | 10 | 15% | 4 | 12% | 6 | 15% |
| Don’t know | 5 | 7% | 2 | 6% | 3 | 8% |
| Prefer not to answer | 8 | 11% | 4 | 13% | 4 | 10% |
Most probands (26 of 31) reported personal experiences with genetic testing. Clinical testing was reported most often, as either germline or tumor testing, around the time of cancer diagnosis. Other experiences with genetic testing included clinical testing for other familial conditions (e.g., Duchenne’s muscular dystrophy) or non-clinical,direct-to-consumer testing. Some participants (6 out of 30) mentioned a family member’s experience with genetic testing, such as a sister who had been tested for pathogenic variants in the BRCA1 gene.
Most relatives (29 of 39) also reported previous experiences with genetic testing, most commonly clinical testing (16 of 29) because of their family member’s cancer diagnosis. Of the 16 relatives who received clinical testing, 9 received a negative result, 4 received a positive result, and 3 were unclear about their results (possibly variants of unknown significance, but the participants may not have remembered). Seven of the 39 relatives reported having participated in direct-to-consumer genetic testing such as Ancestry.com or 23andMe, while 3 reported being Geisinger MyCode® participants. MyCode® is a community DNA sequencing initiative at Geisinger Health System[29.
Preferred messages and experiences
The top preferred messages for probands and relatives were about genetic testing being free to relatives for a given time period after a proband receives a positive result, how ovarian cancer runs in families, what actions can be taken after a positive result, and how genetic testing can help family members (Table 2). Additionally, relatives identified information about how gene variants can increase the risk of ovarian cancer as a preferred message. Preferred modes for both probands and relatives included speaking one-on-one with a doctor, receiving a call from their doctor’s office, seeing a poster in the waiting room, and receiving personalized information in a portal message. Participants preferred personalized communications from a doctor, nurse, or genetic counselor, with the ability to have back-and-forth interaction and ask questions. When asked to clarify the preference for one-on-one conversations with their doctor, participants indicated they were willing to talk to any knowledgeable, trustworthy clinician associated with their doctor or health system, including other clinicians such as nurses or genetic counselors. The critical piece was not their specific doctor or doctors in general, but the ability to have an interactive conversation with a knowledgeable healthcare team member. Phone calls and portal messages were preferred as follow-up from a previous conversation rather than the initial communication. Relatives expressed preference for a video as a form of communication, especially as a complement to a doctor conversation, and a portal message over a mailed letter. We identified five distinct preferred communication experiences for traceback programs (Table 3): Labeled as Alpha, Beta, Gamma, Delta, and Epsilon. All but one experience involved a conversation with a doctor. The Alpha experience involves starting the conversation about genetic testing for ovarian cancer risk first through a discussion between a doctor and the patient followed by communication that is either directed (sent to specific people) or passive (shared in a public area). The Beta experience is characterized by communication within a clinical office setting, either through passive messaging or a clinical discussion, follow by directed communication. The Gamma experience begins with directed communication paired with a conversation with the doctor, followed by passive communication. Both the Delta and Epsilon experiences involve contacting the patient first through both passive and directed messaging; but only Delta includes follow-up via a patient-doctor discussion.
Table 2:
Preferred messages and modes
| All Sites (N=70), N (%) | Probands (N=31) N (%) | Relatives (N=39) N (%) | ||||
|---|---|---|---|---|---|---|
| Preferred messages | ||||||
| Genetic testing is free to family members for 90 days after the ovarian cancer patient is tested. | 42 | 60% | 22 | 71% | 20 | 51% |
| Ovarian cancer runs in families. | 37 | 53% | 19 | 61% | 18 | 47% |
| If you have a genetic variant (change) that increases your ovarian cancer risk, doctors can help. You may be offered cancer screening or prevention options. | 31 | 44% | 19 | 61% | 12 | 31% |
| Genetic testing can find gene variants (changes) that increase your risk of ovarian cancer. | 31 | 44% | 12 | 39% | 19 | 49% |
| If someone in your family ever had ovarian cancer, genetic testing can help other family members. It can help even if the person had ovarian cancer a long time ago. | 29 | 41% | 13 | 42% | 16 | 41% |
| A genetic counselor can talk with you. The counselor can explain genetic testing and answer your questions. | 26 | 37% | 12 | 39% | 14 | 36% |
| People with ovarian cancer and their family members should get genetic testing. A genetic counselor can tell you which family members need genetic testing. | 23 | 33% | 13 | 42% | 10 | 26% |
| Your privacy will be protected. | 21 | 30% | 8 | 26% | 13 | 33% |
| Ovarian cancer is severe | 19 | 27% | 8 | 26% | 11 | 28% |
| If you have a mother or sister with ovarian cancer, this means you have a “family history” of ovarian cancer. Your chance of getting ovarian cancer is higher than the average person if you have this family history. About 5 in 100 women with family history will develop ovarian cancer during their life. (Relatives only) | 17 | 24% | N/A | N/A | 17 | 44% |
| When you get your genetic test results, a doctor may suggest your relatives also get genetic testing. This is called cascade testing | 16 | 23% | 9 | 29% | 7 | 18% |
| If you had genetic testing before 2014, you may need a newer genetic test | 15 | 21% | 11 | 35% | 4 | 10% |
| Your risk is highest if your mother or sister with ovarian cancer has a genetic link (variant) that caused their cancer. (Relatives only) | 15 | 21% | N/A | N/A | 15 | 38% |
| It is important that your family members get genetic testing also. This is important even if you do not get genetic testing. | 12 | 17% | 5 | 16% | 7 | 18% |
| Please talk with a genetic counselor. The genetic counselor can tell you if you need genetic testing, | 10 | 14% | 3 | 10% | 7 | 18% |
| Preferred modes | ||||||
| Clinician communication | ||||||
| Doctor - in person | 64 | 91% | 29 | 94% | 35 | 90% |
| Doctor - phone | 41 | 59% | 22 | 71% | 19 | 49% |
| Directed communication | ||||||
| Patient portal | 32 | 46% | 11 | 35% | 21 | 54% |
| Video | 30 | 43% | 12 | 39% | 18 | 46% |
| Letter to proband | 26 | 37% | 16 | 52% | 10 | 26% |
| Letter to family | 20 | 29% | 2 | 6% | 18 | 46% |
| Infographic | 20 | 29% | 9 | 29% | 11 | 28% |
| Text message | 19 | 27% | 7 | 23% | 12 | 31% |
| Passive communication | ||||||
| Poster - waiting room | 39 | 56% | 18 | 58% | 21 | 54% |
| Screensaver - waiting room | 24 | 34% | 10 | 32% | 14 | 36% |
| Ad (TV) | 17 | 24% | N/A | N/A | 17 | 44% |
| Ad (online) | 12 | 17% | N/A | N/A | 12 | 31% |
| Ad (radio) | 12 | 17% | N/A | N/A | 12 | 31% |
| Public - poster | 9 | 13% | N/A | N/A | 9 | 23% |
Table 3:
Preferred communication experiences
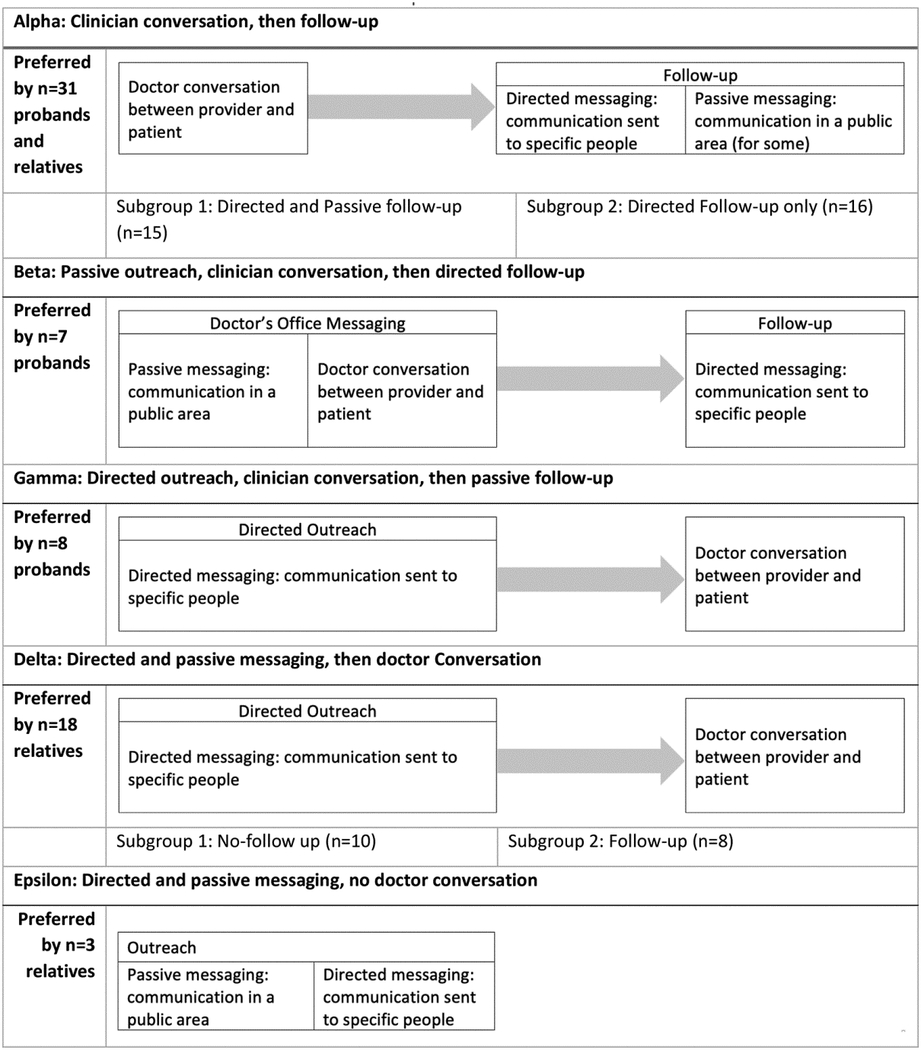
|
We observed some variation across sites. The Alpha experience (doctor conversation with follow-up) was preferred at KPMAS and Geisinger but not KPWA. KPWA probands preferred outreach followed by a doctor conversation and follow-up (Beta and Delta experiences). Privacy was mentioned as a particularly important message by KPMAS participants, who were primarily Black. Most participants said they would participate in traceback genetic testing with their preferred experience, saying their preferences would be the same whether they were contacted at the time of diagnosis or years later. Some participants suggested that the messages might need to evolve over time; but the modes would likely remain constant. Some participants with no living relatives wanted to know what genetic testing would do for them, as helping the next generation was not relevant to them. No message or mode was identified as a dealbreaker, meaning that there were no specific messages or modes that would prompt otherwise-willing participants to decline genetic testing.
Taken together, the preferred experiences and thematic analysis suggest several general requirements for communications about traceback programs (Table 4). First, personalized communication with access to a doctor for further conversation was by far the most preferred experience. Participants considered doctors to be reliable sources of information and guidance, and to be trust-worthy and familiar. As one participant stated, “My doctor is more important to me…He could tell me to jump off a building, and I’d probably say: Are you sure? Because I trust the doctors that I have.” [GE02; proband]
Table 4:
General requirements and exemplar quotes
| Theme | Exemplar quotes |
|---|---|
| Personalized communication with access to physician conversation | “I think just the doctor explains it, the provider explains it, and they might even hand you a little packet and show you what’s inside. Here is what we talked about in maybe an infographic arrangement, and then a letter reminder for you and a letter to open a conversation with your family members who might benefit from the testing. Have it all in one fell swoop there.” [GE03 Proband] “I know it’s snail mail. But nobody, a lot of people don’t check their portal for messages unless it pops up on my phone…I still think that having a generic letter is helpful. If the doctor hasn’t heard from them then having the office reach out and saying, hey. It could even be a video chat, you don’t actually have to come in the office, because the doctor would like to chat with you…about potential risks.” [KPWA4 R] |
| Multiple modes of outreach | “I think if you got a little bit of an understanding from the screen saver and the poster, and then maybe a better understanding from the video, then when the doctor approached you, you’d have a pretty good understanding of it, and will be able to ask questions that you might have.” [GE01 Proband] “I just, it’s like it’s a kind of like a slow reminder, like here you get the folder in the mail. You look at it. Like, oh, okay, or whatever and I throw it away. Then I get a text message and I’m like, man, okay, they really want me to talk to them. Okay. And then I get a phone call. I’m like, okay, okay, I’ll do it [get genetic testing].” [KPWA3R] |
| Messaging focused on clinical benefits to relatives, rationale for testing, and ease of access to testing | “One thing is that your family member has a genetic mutation, and you may be carrying the mutation, too. And if you are carrying it, then what is the risk, or what are the chances that you carry it, and how much is your risk? I guess you also have to say if you don’t carry if, you can still get the disease. It doesn’t mean you are immune to the disease. And how is the testing done? Does it need a blood test? How much does it cost? And will getting the testing done affect your insurance or ability to get insured?” [KPMA4 Pro] “Well, I actually just had a thought. I think promotions like if you’re doing a TV ad type thing, I think famous people that have been through it gets your attention. You know. You know, like who was it the guy, Chadwick guy? The actor, I mean in that case he’s so young and he was so young and stuff and that gets, I think the star’s experiences with these things, I think it helps. Because it, you know, and then even them going and explaining that, hey, for genetic testing it’s just spitting in a cup, not anything else, spit in the thing. No big deal, you know. So, I think some people can shy away from it if they think it’s this big scary test or something. … Yeah, so maybe one of the stars that had ovarian cancer in the family and got genetic testing and they found out that it is a factor, or it is not a factor or whatever. I think adding that kind of thing to an ad, a TV ad would help, be helpful. … It personalizes it.” [KPWA1R] |
Participants also valued being able to ask questions and have them answered and to learn from the doctor if genetic testing is important for them. They felt it would be an efficient process because the doctor could order the test immediately. Per one participant, “Talk to the doctor when you have your appointment and then just do [the genetic testing] then. Especially if it’s not a drawn-out test, I mean, it’s just a blood test. The doctor can order that right there.” [KPWA8P; proband]
Second, participants preferred multiple modes of outreach, both to reach people with their convenience in mind, and to increase the chance of reaching probands and relatives. A participant opined that “any way you can get the information through, go ahead, post it, just poster it, I don’t care, just get it in bulletin boards, I don’t care – get the information out.” [GE21; relative] Participants clearly recognized the clinical value of testing and thought that persistence was justified. “To keep seeing it over and over again,” one participant explained, “and keep being asked about it, and giving you information and reference tools will help people like me, who are procrastinators.” [KPMA10Pro; proband]
Third, messaging should center around the clinical benefits of testing, particularly for relatives; the rationale for testing; and emphasis on how to access testing. As one participant explained, “You don’t hear in everyday life about genetic testing other than the Ancestry DNA…it doesn’t educate you on the fact that [genetic testing] can let you know that you have family history or you’re at risk of certain illnesses and diseases.” [GE07; relative] In our study, the messaging around free testing was the most preferred; this may not be the case in all traceback programs, but it seems pertinent that ease of access and cost is top of mind for participants. As one participant shared:
“I think the genetic testing being free is important because I do believe many people just shy away from doing this because of the expense of it or the fear that it is going to send up a red flag to their insurance company. I know from working in a lab for 34 years genetic testing is quite expensive, so I think that’s important for someone to know, so they don’t have fear about their insurance or having to pay something out of pocket for it.”
[GE24; relative]
Discussion/Conclusion
We conducted a qualitative study utilizing human-centered design methods to determine communication preferences for traceback genetic testing programs among people with a previous diagnosis of ovarian, fallopian tube, or peritoneal cancers and a separate, unrelated sample of people with a documented family history of these cancers. We found the principles that should guide communications around traceback programs include the use of directed, personalized communication and the use of multiple modes of outreach including a conversation with a trusted doctor or other clinician. Preferred messaging emphasizes the rationale for cascade genetic testing in families, the availability of testing, and the clinical benefit of testing to relatives.
Our findings align with other studies in genetic testing indicating patient preference for informational messages about the rationale and benefits of genetic testing. A Delphi study of the information needs of people with breast or ovarian cancer including both health professionals and patients undergoing BRCA1/BRCA2 testing, found that key messages included dominant inheritance patterns, the availability of testing, the importance of pretest counseling, increased risk of breast and ovarian cancer, and the option of risk-reducing mastectomy and bilateral salpingo-oophorectomy[30]. We also uncovered information needs consistent with a recent study of ovarian cancer patients with genetic testing and online community posts[31]. Specifically, patients wanted to receive information that is relevant, understandable, concise, usable, appropriate, sympathetic, and available when needed and through multiple communication modes (i.e., digital technologies, print, and conversations with health care providers)[31]. Furthermore, a similar study to optimize communication with individuals with familial hypercholesterolemia found that messages stressing the health threat to relatives and their susceptibility due to heredity were important motivators for cascade testing[32]. However, a novel finding in our study was the request from probands with no living relatives for messaging showing what genetic testing could do for them.
The different preferences and multiple modes of communication identified supports other findings from the literature that suggests no “one-size-fits-all” communication solution exists and multiple modes of communication with repetitive outreach are necessary for maximum reach[31]. We also confirmed personalization and clinical conversation are important preferences for people receiving genetic testing for hereditary cancer risk, whose individual and family circumstances vary greatly[31,33,34]. Our participants preferred some form of communication with their physician as the starting point for discussion. While standardized family communication aids are well studied and acceptable information sources to families[35], they may be most effective in at-risk relative notification as a complement to direct clinician communication[34,36]. Animated videos may provide a way to communicate complex information, serving as reference material and can complement a clinician conversation[37]. However, as suggested by our results and others, clinician communication may be critical to the process. Additional work is necessary to design a traceback genetic testing process which provides an appropriate amount of clinician communication in a scalable, economically viable way.
The human-centered design approach identified important information to be used in traceback programs, including the clinical benefits for traceback programs and cascade genetic testing for relatives with and without ovaries. Notably, though we did not ask any questions directly about this, multiple participants were surprised to learn from our materials that all close relatives should have the opportunity to receive genetic testing for hereditary ovarian cancer, not just people with ovaries. They were unaware that having a pathogenic or likely pathogenic variant in a gene such as BRCA1 or BRCA2, which puts someone at greater risk for ovarian cancer, also increases risk for many other cancers even if they do not have ovaries. Additionally, participants were surprised to learn anyone who is BRCA-positive can pass on the pathogenic or likely pathogenic variant to their biological children, which increases their risk for ovarian and other cancer.
We found that among subsets of our interviewed population, specifically Black or African-American participants, privacy was a very important consideration. Similar findings, along with distrust and discrimination concerns, have been shown in the literature[38–41].
Our study contributes data collected using human-centered design methods on a specific subtype of cascade testing: retrospective traceback programs involving relatively unexpected outreach to cancer survivors, in some cases years after the initial diagnosis. We set out to explore the “surprise” nature of traceback programs where people are contacted after time has passed since a diagnosis; or for relatives, completely out of the blue. Though it is difficult to compare directly across studies, our findings suggest personalized outreach and quick access to a clinician conversation may be particularly important, and the ways in which relatives can access genetic testing might be relevant messaging in a traceback setting. Early efforts at traceback programs have successfully reached probands and at-risk relatives, though these programs may be time and resource intensive[5,42]. Our work identifies patient and family communication preferences which suggest ways to efficiently reach families using tested messaging. To the best of our knowledge, this study is the first use of human-centered design methodologies to inform the design and implementation of retrospective traceback programs.
Limitations
The interviews were conducted remotely, over the phone or via video, due to the COVID-19 pandemic. The conversations may have been different if the participants were in person, interacting with the materials directly with the interviewers. However, offering phone and video interviews may have improved recruitment due to the convenience to the participants. Another limitation was a lack of probands and relatives who had received genetic testing which returned a pathogenic variant. This limited our ability to obtain information about real-world situations in which a proband had approached their relatives to encourage genetic testing. While the KPMAS participants were selected for Black race and Geisinger serves a predominantly rural population, the findings of this study alone are not sufficient to draw generalized conclusions about preferences among different racial and ethnic groups or other underserved populations. In addition, no participants were recruited outside of an existing system of care, limiting the captured experiences and opinions to individuals that were members of a healthcare system. Non-English speakers were also not included. No transgender patients participated, though they were not specifically excluded.
Conclusion
In this multi-site qualitative study, patients and relatives preferred personalized communication strategies, delivered by clinicians, with a variety of outreach modes, for traceback genetic testing programs. The results of this qualitative study are informing the design of patient-centered programs to be implemented in the respective health systems, focused on encouraging and facilitating traceback cascade genetic testing in individuals with a personal or family history of ovarian, peritoneal, or fallopian tube cancer[6].
Supplementary Material
Acknowledgements
The authors thank Dr. Anna Baker for her help conducting interviews for this research study.
Funding Sources
This research was funded by the National Institutes of Health, grant number 1U01CA240747-01A1.
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Statement of Ethics
This study protocol was reviewed and granted exemption from requiring ethics approval and written informed consent by the Geisinger Institutional Review Board, Geisinger Health System (IRB #2020–0281) therefore written informed consent was not obtained. Eligible participants provided oral consent to participate both in the recruitment call, as well as before the recording and participating in the interview.
Publisher's Disclaimer: Disclaimer:
Publisher's Disclaimer: Accepted, unedited article not yet assigned to an issue. The statements, opinions and data contained in this publication are solely those of the individual authors and contributors and not of the publisher and the editor(s). The publisher and the editor(s) disclaim responsibility for any injury to persons or property resulting from any ideas, methods, instructions or products referred to the content.
Data Availability Statement
Data obtained or analyzed during this study are included in this article and/or its online supplementary files. Further inquiries can be directed to the corresponding author.
References
- 1.varian Cancer — Cancer Stat Facts [Internet]. [cited 2021 Dec 17]. Available from: https://seer.cancer.gov/statfacts/html/ovary.html
- 2.American Cancer Society. Special Section: Ovarian Cancer. Cancer Facts & Figures 2018 [Internet]. 2018. [cited 2021 Dec 22];28–43. Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-special-section-ovarian-cancer-2018.pdf [Google Scholar]
- 3.NCCN Clinical practice guidelines in oncology. genetic/familial high risk assessment: breast and ovarian version 2.2019. NCCN Guidelines; 2018. [Google Scholar]
- 4.McCuaig JM, Stockley TL, Shaw P, Fung-Kee-Fung M, Altman AD, Bentley J, et al. Evolution of genetic assessment for BRCA-associated gynaecologic malignancies: a Canadian multisociety roadmap. J Med Genet. 2018. Sep 1;55(9):571–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delahunty R, Nguyen L, Craig S, Creighton B, Ariyaratne D, Garsed DW, et al. TRACEBACK: Testing of Historical Tubo-Ovarian Cancer Patients for Hereditary Risk Genes as a Cancer Prevention Strategy in Family Members. J Clin Oncol [Internet]. 2022. Mar 9 [cited 2022 Mar 31];JC02102108. Available from: https://pubmed.ncbi.nlm.nih.gov/35263119/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinucci A, Henrikson NB, Jonas MC, Basra S, Blasi P, Brown J, et al. Feasibility and Assessment of a Cascade Traceback Screening Program (FACTS): Protocol for a Multisite Study to Implement and Assess an Ovarian Cancer Traceback Cascade Testing Program. J Pers Med. 2021. Jun 1;11(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasmussen LV, Overby CL, Connolly J, Chute CG, Denny JC, Freimuth RR, et al. Practical considerations for implementing genomic information resources: Experiences from eMERGE and CSER: Experiences from eMERGE and CSER. Appl Clin Inform. 2016;7(3):870–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rafi I, Crinson I, Dawes M, Rafi D, Pirmohamed M, Walter FM. The implementation of pharmacogenomics into UK general practice: a qualitative study exploring barriers, challenges and opportunities. J Community Genet. 2020. Jul 1;11(3):269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall LK, Kunz BF, Davis EV, Dawson RI, Powers RS. The Cancer Experience Map: An Approach to Including the Patient Voice in Supportive Care Solutions. J Med Internet Res. 2015. May;17(5):e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matheson GO, Pacione C, Shultz RK, Klügl M. Leveraging Human-Centered Design in Chronic Disease Prevention. Am J Prev Med. 2015. Apr 1;48(4):472–9. [DOI] [PubMed] [Google Scholar]
- 11.Silva H, Gonzaga do Nascimento M, de Morais Neves C, Oliveira I, Cipolla C, Batista de Oliveira G, et al. Service blueprint of comprehensive medication management: A mapping for outpatient clinics. Res Social Adm Pharm [Internet]. 2021. Jan 24 [cited 2021 Jul 15];17(10):1727–36. Available from: https://pubmed.ncbi.nlm.nih.gov/33558157/ [DOI] [PubMed] [Google Scholar]
- 12.Kelley D, Moggridge B, Nuttall M. Design Kit: The Field Guide to Human-Centered Design. 2009;189. [Google Scholar]
- 13.Kelley T, Kelley D. Creative Confidence, Crown Business. Crown Publishing Group; ), New York; 2013. [Google Scholar]
- 14.Martin B, Hanington BM. Universal methods of design expanded and revised: 125 Ways to research complex problems, develop innovative ideas, and design effective solutions. 1st ed. Beverly, MA: Rockport Publishers; 2019. 207 p. [Google Scholar]
- 15.Schuler D, Namioka A, editors. Participatory design: Principles and practices. 1st ed. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc.; 1993. 334 p. [Google Scholar]
- 16.Ross TR, Ng D, Brown JS, Pardee R, Hornbrook MC, Hart G, et al. The HMO Research Network Virtual Data Warehouse: A Public Data Model to Support Collaboration. EGEMS (Wash DC). 2014. Mar 24;2(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Community Advisory Board – Mid-Atlantic Permanente Research Institute [Internet]. [cited 2022 Sep 26]. Available from: https://mapri.kaiserpermanente.org/collaborate-with-us/cab/
- 18.Holtzblatt K, Beyer H, editors. Contextual Design: Design for Life [Internet]. 2nd ed. Contextual Design. Burlington, MA: Morgan Kauffman Books; 2017. [cited 2023 Jan 12]. 446 p. Available from: http://www.sciencedirect.com:5070/book/9780128008942/contextual-design [Google Scholar]
- 19.Rasinski KA, Lee L, Krishnamurty P. Question order effects. APA handbook of research methods in psychology, Vol 1: Foundations, planning, measures, and psychometrics. 2012. Mar 6;229–48. [Google Scholar]
- 20.Stowell E, O’Leary TK, Kimani E, Paasche-Orlow MK, Bickmore T, Parker AG. Investigating Opportunities for Crowdsourcing in Church-Based Health Interventions: A Participatory Design Study. Conference on Human Factors in Computing Systems - Proceedings [Internet]. 2020. Apr 21 [cited 2022 Jan 27];1–12. Available from: 10.1145/3313831.3376833 [DOI] [Google Scholar]
- 21.Miro | Online Whiteboard for Visual Collaboration [Internet]. [cited 2021 Dec 22]. Available from: https://miro.com/
- 22.Beebe J Rapid qualitative inquiry: A field guide to team-based assessment. 2nd ed. Rowman & Littlefield Pub. Inc.; 2014. [Google Scholar]
- 23.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009. Apr 1;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019. Jul 1;95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scupin R The KJ method: A technique for analyzing data derived from Japanese ethnology. Hum Organ. 1997;56(2):233–7. [Google Scholar]
- 26.Watkins DC. Rapid and Rigorous Qualitative Data Analysis: The “RADaR” Technique for Applied Research. 10.1177/1609406917712131. 2017. Jun 8;16(1). [DOI] [Google Scholar]
- 27.Creswell JW, Poth CN. Qualitative inquiry & research design : choosing among five approaches. Fourth. SAGE Publications, Inc.; 2017. 459 p. [Google Scholar]
- 28.Hennink M, Kaiser BN. Sample sizes for saturation in qualitative research: A systematic review of empirical tests. Soc Sci Med. 2022. Jan 1;292:114523. [DOI] [PubMed] [Google Scholar]
- 29.Carey DJ, Fetterolf SN, Davis FD, Faucett WA, Kirchner HL, Mirshahi U, et al. The Geisinger MyCode community health initiative: an electronic health record-linked biobank for precision medicine research. Genet Med [Internet]. 2016. Sep 1 [cited 2022 Sep 27];18(9):906–13. Available from: https://pubmed.ncbi.nlm.nih.gov/26866580/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobs C, Pichert G, Harris J, Tucker K, Michie S. Key messages for communicating information about BRCA1 and BRCA2 to women with breast or ovarian cancer: Consensus across health professionals and service users. Psychooncology [Internet]. 2017. Nov 1 [cited 2022 Jul 28];26(11):1818–24. Available from: https://pubmed.ncbi.nlm.nih.gov/28101941/ [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Yi S, Trace CB, Williams-Brown MY. Understanding the Information Needs of Patients With Ovarian Cancer Regarding Genetic Testing to Inform Intervention Design: Interview Study. JMIR Cancer [Internet]. 2022. Jan 1 [cited 2022 Jul 28];8(1). Available from: https://pubmed.ncbi.nlm.nih.gov/35133282/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell-Salome G, Walters NL, Ladd IG, Sheldon A, Ahmed CD, Brangan A, et al. Motivating cascade testing for familial hypercholesterolemia: applying the extended parallel process model for clinician communication. Transl Behav Med [Internet]. 2022. Jul 18 [cited 2022 Jul 28];12(7):800–9. Available from: https://academic.oup.com/tbm/article/12/7/800/6569252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.di Pietro ML, Zaçe D, Orfino A, di Raimo FR, Poscia A, de Matteis E, et al. Intrafamilial communication of hereditary breast and ovarian cancer genetic information in Italian women: towards a personalised approach. Eur J Hum Genet [Internet]. 2021. Feb 1 [cited 2022 Jul 28];29(2):250–61. Available from: https://pubmed.ncbi.nlm.nih.gov/32929237/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baroutsou V, Underhill-Blazey ML, Appenzeller-Herzog C, Katapodi MC. Interventions Facilitating Family Communication of Genetic Testing Results and Cascade Screening in Hereditary Breast/Ovarian Cancer or Lynch Syndrome: A Systematic Review and Meta-Analysis. Cancers (Basel) [Internet]. 2021. Feb 2 [cited 2022 Jul 28];13(4):1–25. Available from: https://pubmed.ncbi.nlm.nih.gov/33672149/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ratnayake P, Wakefield CE, Meiser B, Suthers G, Price MA, Duffy J, et al. An exploration of the communication preferences regarding genetic testing in individuals from families with identified breast/ovarian cancer mutations.Fam Cancer [Internet]. 2011. Mar [cited 2022 Jul 28];10(1):97–105. Available from: https://pubmed.ncbi.nlm.nih.gov/20878485/ [DOI] [PubMed] [Google Scholar]
- 36.Garcia C, Sullivan MW, Lothamer H, Harrison KM, Chatfield L, Thomas MH, et al. Mechanisms to increase cascade testing in hereditary breast and ovarian cancer: Impact of introducing standardized communication aids into genetic counseling. J Obstet Gynaecol Res [Internet]. 2020. Sep 1 [cited 2022 Jul 28];46(9):1835–41. Available from: https://pubmed.ncbi.nlm.nih.gov/32656916/ [DOI] [PubMed] [Google Scholar]
- 37.Aeilts AM, Carpenter KM, Hovick SR, Byrne L, Shoben AB, Senter L. The impact of a cascade testing video on recipients’ knowledge, cognitive message processing, and affective reactions: A formative evaluation. J Genet Couns [Internet]. 2021. Jun 1 [cited 2022 Jul 28];30(3):656–64. Available from: https://pubmed.ncbi.nlm.nih.gov/33142025/ [DOI] [PubMed] [Google Scholar]
- 38.Suther S, Kiros GE. Barriers to the use of genetic testing: A study of racial and ethnic disparities. Genetics in Medicine 2009 11:9 [Internet]. 2009. Sep [cited 2022 Jul 28];11(9):655–62. Available from: https://www.nature.com/articles/gim200997 [DOI] [PubMed] [Google Scholar]
- 39.Sutton AL, He J, Tanner E, Edmonds MC, Henderson A, de Mendoza AH, et al. Understanding Medical Mistrust in Black Women at Risk of BRCA 1/2 Mutations. J Health Dispar Res Pract [Internet]. 2019. [cited 2022 Jul 28];12(3):35. Available from: /pmc/articles/PMC7521839/ [PMC free article] [PubMed] [Google Scholar]
- 40.Sanderson SC, Brothers KB, Mercaldo ND, Clayton EW, Antommaria AHM, Aufox SA, et al. Public Attitudes toward Consent and Data Sharing in Biobank Research: A Large Multi-site Experimental Survey in the US. The American Journal of Human Genetics. 2017. Mar 2;100(3):414–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peters N, Rose A, Armstrong K. The Association between Race and Attitudes about Predictive Genetic Testing. Cancer Epidemiology, Biomarkers & Prevention [Internet]. 2004. Mar 1 [cited 2022 Jul 28];13(3):361–5. Available from: https://aacrjournals.org/cebp/article/13/3/361/256599/The-Association-between-Race-and-Attitudes-about [PubMed] [Google Scholar]
- 42.Weinmann S, Phillips S, Sweet K, Cosgrove CM, Senter L. Hospital-based ovarian cancer patient traceback program results in minimal genetic testing uptake. Gynecol Oncol [Internet]. 2022. Mar 1 [cited 2022 Jul 28];164(3):615–21. Available from: https://pubmed.ncbi.nlm.nih.gov/34998598/ [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data obtained or analyzed during this study are included in this article and/or its online supplementary files. Further inquiries can be directed to the corresponding author.


