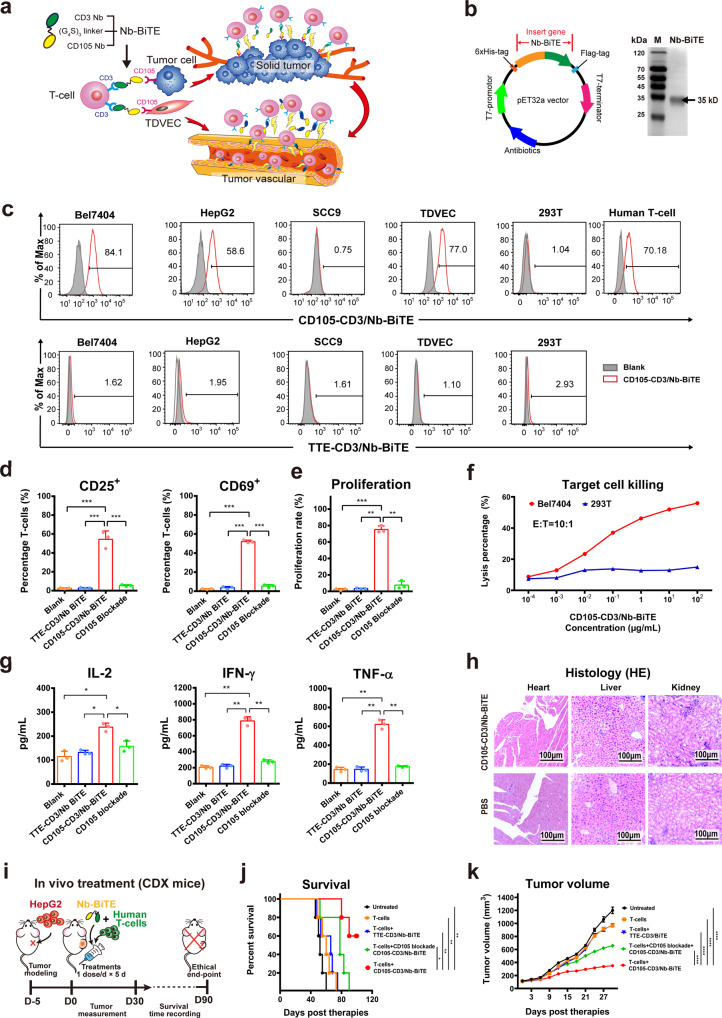
Fig. 1.
CD105-CD3/Nb-BiTE enhances the anti-tumor T-cell activities in vitro and in vivo, through specifically bridging and redirecting T-cells to CD105-expressing target cells. a The schematic illustration of the construction and the mechanism of CD105-CD3/Nb-BiTE. CD105-CD3/Nb-BiTE consists of two nanobodies (Nbs) specific to human CD3 of T-cells and CD105 of tumor cells connected with a triplicate Gly4Ser (Gly4Ser)3 flexible linker, and directs T-cells to target the CD105-overexpressing tumor cells and tumor vessels. b The schematic diagram of molecular structure of recombinant plasmid of Nb-BiTE (left panel) and the gel image of western blot assay for the imidazole-eluted CD105-CD3/Nb-BiTE fusion protein (~35 kDa molecular weight) (right panel). c Representative graphs of flow cytometry data showing binding specificity of CD105-CD3/Nb-BiTE to different cell types. An isotypic TTE-CD3/Nb-BiTE served as a nonspecific control. d CD105-CD3/Nb-BiTE increased the expression of CD25 and CD69 and activated T-cells, determined by PE-conjugated anti-hCD25 and –CD69 mAbs (eBioscience, USA). e CD105-CD3/Nb-BiTE promoted proliferation of human T-cells, determined by pre-staining of PKH26 dye (Sigma-Aldrich, USA). f CD105-CD3/Nb-BiTE enhanced specific killing ability of T-cells against CD105high tumor cells (Bel7404) in a dose-dependent manner but not against CD105- cells (293T). g CD105-CD3/Nb-BiTE increased the secretion of anti-tumor cytokines TNF-α, IFN-γ and IL-2 by T-cells under the stimulation of CD105+ tumor cells (HepG2), determined by commercial ELISA kits (R&D Systems, USA). h Representative H&E images of BALB/c mice receiving 10 μg of Nb-BiTE or PBS daily for 5 consecutive days (n = 3) showing no obvious histological difference between the two groups in the heart, and minimal histological differences in the liver and the kidney. Scale bar: 100 μm. i The schematic diagram shows CDX model in NOD/SCID mice and treatment scheme. CD105-CD3/Nb-BiTE (10 μg) and human T-cells (4 × 107) were administered via tail-vein injection for 5 continuous days for each mouse to evaluate the anti-tumor efficacy. j The Kaplan–Meier survival curves of mice receiving treatments until the ethical endpoint (90 days). k The tumor volume profiles of mice receiving treatments in 30 days after the first administration. Statistics were performed using GraphPad Prism software. All data are represented as mean ± SEM of triplicates, from at least two independent experiments. The differences among groups were determined using the ANOVA analysis of variance test by LSD post hoc test. A two-tailed p value of <0.05 was considered statistically significant. Significance: ****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05