Abstract
Study Objectives:
This meta-analysis aimed to investigate the feasibility and effectiveness of continuous positive airway pressure (CPAP) treatment in stroke patients with sleep apnea.
Methods:
PubMed, EMBASE, and the Cochrane Library were searched from inception until July 28, 2022, for randomized controlled trials comparing the use of CPAP and usual treatment in patients with stroke or transient ischemic attack and sleep apnea. The primary outcome measures were the feasibility of CPAP therapy, neurological function, and functional status.
Results:
After screening 5,747 studies, 14 studies with 1,065 patients were included in this meta-analysis. Overall, 8 of the 14 studies recorded CPAP use, and the mean CPAP use was 4.47 hours per night (95% confidence interval [CI]: 3.85–5.09). The risk ratio of discontinuing CPAP was 1.50 (95% CI: 0.76–2.94; P = .24). Analysis of the neurofunctional scales showed that CPAP treatment improved neurological function (standardized mean difference: 0.28; 95% CI: 0.02–0.53), but there was substantial heterogeneity (I2 = 57%, P = .03) across the studies. CPAP treatment had no significant effect on functional status vs the control (standardized mean difference: 0.25; 95% CI: −0.01 to 0.51), but the studies also had substantial heterogeneity (I2 = 55%, P = .06).
Conclusions:
CPAP treatment is feasible in patients with stroke and sleep apnea and may improve neurological outcomes in these patients. However, this finding should be interpreted with caution because of the substantial heterogeneity of current trials.
Citation:
Fu S, Peng X, Li Y, Yang L, Yu H. Effectiveness and feasibility of continuous positive airway pressure in patients with stroke and sleep apnea: a meta-analysis of randomized trials. J Clin Sleep Med. 2023;19(9):1685–1696.
Keywords: stroke, sleep apnea, continuous positive airway pressure, meta-analysis
BRIEF SUMMARY
Current Knowledge/Study Rationale: Sleep apnea is a recognized independent risk factor for stroke; it can lead to worse clinical outcomes, longer hospital stays, increased long-term mortality, and risk of recurrent stroke. As the standard treatment modality for sleep apnea, continuous positive airway pressure (CPAP) can reduce disease severity and improve sleep-related quality of life in the majority of patients with sleep apnea, but its effectiveness in patients with stroke and sleep apnea remains controversial.
Study Impact: This study indicates that CPAP is a feasible treatment for patients with stroke and sleep apnea, and CPAP is beneficial for neurological improvement in these patients. These results offer evidence for the inclusion of CPAP treatment in the rehabilitation program for patients with stroke and sleep apnea.
INTRODUCTION
Sleep apnea (SA) is a common sleep disorder characterized by repeated complete (apnea) or partial collapse (hypopnea) of the upper airway during sleep. These events last at least 10 seconds. Stroke is an acute brain disease caused by sudden rupture of blood vessels in the brain or by ischemia of the brain due to blockage of blood vessels. Stroke has high rates of incidence, mortality, and disability. There is also a causal and bidirectional relationship between stroke and SA, with SA recognized as an independent risk factor for stroke.1 SA is prevalent in as many as 30–82.7% of patients with stroke or transient ischemic attack (TIA).2,3 Furthermore, stroke may also cause new-onset SA or worsen pre-existing SA.4 Patients with stroke and SA are at higher risk of early neurological worsening,5 longer hospital stays,6 and higher long-term mortality and recurrent stroke.7–9
SA is amenable to treatment and is considered a modifiable stroke risk factor.10 However, unlike common conventional risk factors for recurrent stroke diagnosed and treated after admission (eg, hypertension, diabetes mellitus, and hyperlipidemia), SA is often overlooked.10 One survey revealed a low level of awareness among health professionals and medical students on SA being an established and modifiable risk factor for stroke.11 Additionally, screening for classic symptoms of SA and formal testing for SA after stroke are rare.12 Notably, secondary stroke prevention guidelines by the American Heart Association/American Stroke Association recommended that patients with ischemic stroke and TIA undergo screening and treatment for SA.13
Continuous positive airway pressure (CPAP) is the primary treatment modality for SA; it can reduce disease severity, sleepiness, and blood pressure and improve sleep-related quality of life in most patients with SA.14 However, its effectiveness in patients with stroke and SA remains controversial. Published meta-analyses have synthesized the available evidence on the effectiveness of CPAP in patients with stroke and SA and concluded that CPAP was associated with better neurological outcomes in patients with stroke and SA.15,16 However, they included not only randomized controlled trials (RCTs)15, and included RCTs with sham-CPAP as a control group.16 There have been few RCTs with sham-CPAP as a control group due to the difficulty in the implementation of CPAP therapy for double blinding and the problems of low retention rates during the implementation of sham-CPAP. Moreover, previous related studies mainly focused on neurological improvement.15,16 However, the improvement in functional status during rehabilitation after stroke is also crucial. Additionally, several studies aimed at analyzing the effect of CPAP in patients with stroke and SA have been published since the last meta-analyses.
This meta-analysis aimed to comprehensively assess the most recent evidence on the feasibility and effect of CPAP treatment in patients with stroke and SA. Based on previous relevant studies,15,16 the study included only RCTs in which the control group received the usual treatment. Functional status was also included as a primary outcome measure.
METHODS
Data sources and study selection
This meta-analysis of CPAP in patients with stroke and SA was based on the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines.17 We systematically searched PubMed, EMBASE, and the Cochrane Library (from inception until July 28, 2022) to identify all RCTs evaluating the effect of CPAP therapy in patients with stroke and SA. Search terms used a combination of free and MeSH (medical subject heading) terms related to the population (eg, “stroke”, “cerebrovascular disorders”, “sleep apnea syndromes”, “sleep apnea*”) and intervention (eg, “continuous positive airway pressure”, “positive-pressure respiration”). Articles were screened independently by 2 authors, and duplicate publications were removed. We then read the title and abstract of each article, examined whether the full text was available, and excluded articles that did not meet the inclusion criteria. The full texts of the final included articles were read. Studies were included if they met the following criteria: (1) any RCTs, including crossover and cluster RCTs; (2) patients aged > 18 years; (3) stroke/TIA patients with SA that was diagnosed based on clinical suspicion, respiratory polygraphy, auto-CPAP or polysomnography, and 2 forms of SA (obstructive vs unclassified); (4) the experimental group was treated with CPAP; (5) usual treatment was used in the control group; and (6) studies published in any language. RCTs meeting any of the following criteria were excluded: (1) studies designed to explore the prevalence of SA in stroke and (2) RCTs comparing CPAP with other treatments for SA (eg, nasotracheal suction mechanical ventilation).
Outcome measures
The primary outcomes were as follows:
The feasibility of CPAP therapy, which included the use of CPAP (hours/night) during treatment (adherence) and the dropout rate during treatment and follow-up. Adherence was considered acceptable when CPAP was used for ≥ 4 hours/night.
Neurological function, which was measured by the mean change in scores on the neurological function rating scales from baseline to postintervention. These neurological function scales included the National Institute of Health Stroke Scale or the Canadian Neurological Scale.
Functional status was measured as the mean change in the functional scale from baseline to postintervention. These functional scales included the Barthel index (20-point scale and 100-point scale), motor section of Functional Independence Measure, or subscales (self-care) of the Utrecht Scale for Evaluation of Rehabilitation.
The secondary outcomes were as follows:
Incidence of recurrent vascular events during treatment and follow-up (including recurrent TIA, stroke, acute myocardial infarction, hospitalization for congestive heart failure, death, etc).
Cognitive function, measured using the mean change scores in cognitive function rating scales from baseline to postintervention. These cognitive function scales included the Mini-Mental State Examination, Montreal Cognitive Assessment, or Addenbrooke’s Cognitive Examination.
Depression was assessed by measuring the mean change in scores on depression rating scales from baseline to postintervention. These scales included Hospital Anxiety and Depression Scale–Depression subscale, Hamilton Rating Scale for Depression, Beck Depression Inventory-1, Patient Health Questionnaire-9, or Montgomery-Asberg Depression Rating Scale.
Sleepiness was measured using the mean change in the sleepiness scale from baseline to postintervention. These scales included the Epworth Sleepiness Scale or Stanford Sleepiness Scale.
Reduction in apnea-hypopnea index (AHI) was obtained by subtracting the AHI values at baseline from that at the end of treatment.
When 2 or more scales assessing the same function appeared simultaneously in a study, 1 scale was selected based on the described sequence. However, when the data for the selected scale were incomplete, the other 1 scale was chosen also based on the described sequence. Outcome data were extracted at the longest postintervention time point. As the scales used by the studies to assess related functions varied, the posttreatment difference from baseline was used to represent the change in each function. In some neurological assessment scales, higher scores represent better outcomes, whereas in others, lower scores represent better outcomes. Therefore, for the statistical analysis, the data were adjusted by using positive values to represent improved neurological function. For the AHI outcome, a negative difference in the mean change in AHI, represented by negative values, indicated improvement in AHI after CPAP intervention.
Data extraction and quality assessment
Two researchers independently extracted key characteristics of identified studies, which included study characteristics (publication year, first author), patient characteristics (number of participants in each group, sex, mean age, mean body mass index, type of stroke, time since stroke, inclusion of AHI), intervention details (time of intervention), and outcome measures (as outlined above).
According to the recommendations of the Cochrane intervention system evaluation manual,18 2 researchers independently evaluated the methodological quality of the study. Each study was classified as “low,” “high,” or “uncertain” based on the different types of bias, including selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias. The criteria for rating study quality were as follows: high-risk study (≥ 3 items rated as high risk of bias), low-risk study (≥ 5 items rated as low risk and no more than 1 as high risk), and moderate-risk study (all remaining situations). Any differences in opinion between the authors were resolved through discussion and consultation with a third author.
Data analyses
The meta-analysis was conducted using the Der Simonian-Laird random-effects model for each primary and secondary outcome. Outcome data were calculated using the standardized mean difference (SMD) with a 95% confidence interval (CI) for continuous outcomes and risk ratio with a 95% CI for dichotomous outcomes. Statistical significance was set at P < .05. Heterogeneity between studies was assessed using the I2 statistic, with I2 > 50% indicating substantial heterogeneity and I2 > 75% indicating considerable heterogeneity. The effect sizes are presented as forest plots with 95% CIs. Funnel plots and Egger’s regression symmetry tests were used to assess evidence of publication bias, with P < .05 indicating significant publication bias. Subgroup analyses on neurological function and functional status were conducted to examine whether effect estimates were influenced by initiation time of CPAP since stroke, exclusion of previously diagnosed SA, form of sleep apnea, CPAP treatment duration, and sample size of included studies. To clarify possible sources of statistical heterogeneity, sensitivity analyses were performed on neurological function and functional status by excluding studies individually and the heterogeneity and effect size for all of the remaining studies were analyzed. Dichotomous outcome data were managed according to the intention-to-treat principle. RevMan (version 5.4.1; The Cochrane Collaboration, London, United Kingdom), the meta package in R (version 3.3.2; R Foundation for Statistical Computing, Vienna, Austria), and Stata (version 16.0; StataCorp, College Station, TX, USA) were used to organize the data, perform statistical analysis, and generate tables and graphs.
RESULTS
Study selection and characteristics
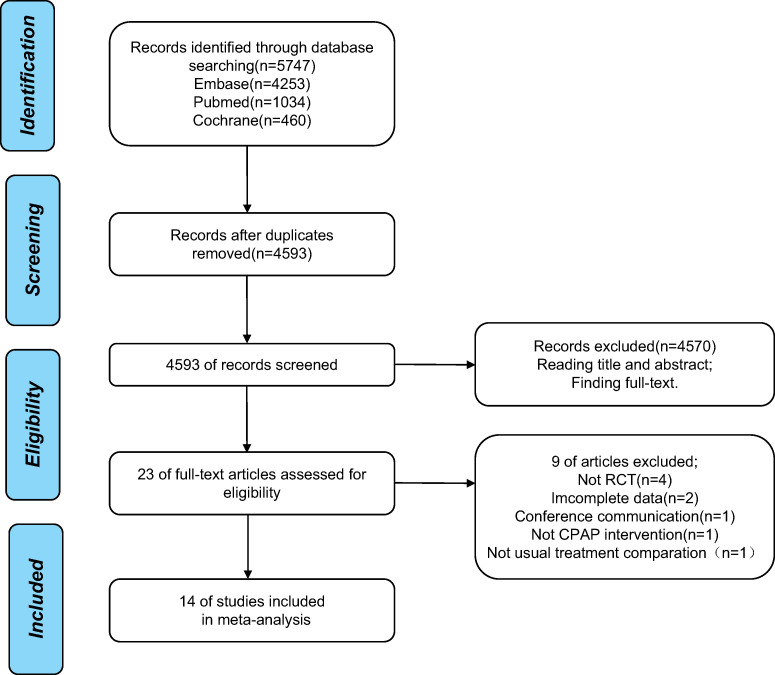
A total of 5,747 articles were retrieved from the literature search; of these, 1,154 were duplicate publications and were excluded. After screening the title and abstract and evaluating the availability of full-text versions, 23 articles were selected. Further, after retrieving and analyzing the full text of the 23 studies, 9 studies were excluded, and 14 clinical trials were finally included in the meta-analysis (Figure 1 illustrates a detailed flow chart of study selection).
Figure 1. Flow chart of study selection.
CPAP = continuous positive airway pressure, RCT = randomized controlled trial.
The 14 RCTs19–32 included in the meta-analysis comprised 1,065 patients with stroke or TIA, with 564 in the intervention group and 501 in the control group. Seven of these studies19,23,25,27–30 did not distinguish between patients with ischemic and hemorrhagic stroke (457 in total), 4 studies20,22,24,32 enrolled patients with ischemic stroke (245 in total), 2 studies26,31 included patients with either ischemic stroke or TIA (293 in total), and 1 study21 included only patients with TIA (70 in total). Seven studies21–23,25,26,29,30 excluded patients who had previously been diagnosed with SA, and patients with SA were randomly divided into groups according to polysomnography before treatment. Five studies19,20,24,27,31 performed polysomnography on patients after inclusion, and these patients were randomly divided into groups. One study28 included patients with confirmed SA. One study32 included patients with highly suspected SA without sleep monitoring. Seven studies22,23,25,27–30 included patients with definitive obstructive sleep apnea (OSA), while the remaining 7 studies19–21,24,26,31,32 did not clearly distinguish between the types of SA. The criteria for AHI at inclusion varied across studies. The sample size of these studies ranged from 25 to 252 participants. The disease course in these patients ranged from the hyperacute phase (within 24 h) to the late phase (6 mo). The study intervention duration ranged between 72 hours and 24 months. In 5 trials,21,22,24,26,32 treatment was initiated within 7 days after stroke (early onset). The remaining studies19,20,23,25,27–31 initiated treatment 7 days to 6 months after the cerebrovascular event (late onset). Table 1 summarizes the detailed characteristics of the study population.
Table 1.
Baseline characteristics of the included studies.
| Study (First Author, Year) | Treatment/Comparation | Age, Mean ± SD, y | Sex (Male/Female), n/n | BMI, Mean ± SD, kg/m2 | Stroke Type* | Time Since Stroke | Inclusion AHI, events/h | Treatment Duration | Dropout Rate (CPAP/UT), % |
|---|---|---|---|---|---|---|---|---|---|
| Sandberg, 200119 | 33/30 | 77.48 ± 7.12 | 29/34 | 24.64 ± 4.41 | Stroke | 14–28 days | ≥15 | 28 days | 20/20 |
| Hsu, 200620 | 15/15 | 73.50 ± 7.44 | 20/10 | 25.95 ± 6.66 | Ischemic stroke | 21–25 days | ≥30 | 56 days | 0/0 |
| Bravata, 201021 | 45/30 | 66.69 ± 12.15 | 35/35 | 28.31 ± 5.06 | TIA | 39.4 ± 23.7 hours | ≥5 | 90 days | 20/0 |
| Parra, 201122 | 71/69 | 64.70 ± 9.20 | 99/41 | 29.50 ± 4.30 | Ischemic stroke | 3–6 days | ≥20 | 730 days | 11/13 |
| Ryan, 201123 | 25/23 | 61.75 ± 11.53 | 35/9 | 28.05 ± 5.54 | Stroke | 21.5 ± 8.7 days | ≥15 | 28 days | 29/0 |
| Minnerup, 201224 | 25/25 | 66.00 ± 10.48 | 20/30 | 27.25 ± 2.84 | Ischemic stroke | 1 day | ≥10 | 8 days | 6/7 |
| Aaronson, 201625 | 20/16 | 59.14 ± 8.64 | 22/14 | 27.08 ± 5.75 | Stroke | 16.8 ± 15.0 days | ≥20 | 28 days | 20/0 |
| Bravata, 201826 | 168/84 | 60.52 ± 12.80 | 149/103 | 30.35 ± 7.77 | Ischemic stroke or TIA | Within 1 week | NG | 365 days | 12/4 |
| Gupta, 201827 | 34/36 | 53.00 ± 11.83 | 57/13 | 25.26 ± 4.07 | Stroke | 6 weeks–6 months | >15 | 90–180 days | 15/13 |
| Wan, 201828 | 25/29 | 59.79 ± 11.22 | 30/19 | NG | Stroke | NG | ≥5 | 90 days | 12/0 |
| Kim, 201929 | 20/20 | 65.10 ± 12.67 | 29/11 | 23.85 ± 3.79 | Stroke | 7 days-6 months | ≥20 | 21 days | 20/0 |
| Ren, 201930 | 49/97 | 61.79 ± 9.97 | 85/61 | 26.84 ± 5.47 | Stroke | Within 4 weeks | ≥20 | 730 days | 0/0 |
| Bernasconi, 202031 | 19/22 | 64.42 ± 6.57 | 32/9 | 28.15 ± 5.35 | Ischemic stroke or TIA | 60–90 days | ≥20 | 730 days | 4/16 |
| Barlinn, 202132 | 15/10 | 67.39 ± 8.5 | 18/7 | 27.32 ± 4.67 | Ischemic stroke | Within 24 hours | NG | 3 days | 16/18 |
Unspecified stroke type means ischemic and hemorrhagic strokes were not distinguished. AHI = apnea-hypopnea index, BMI = body mass index, CPAP = continuous positive airway pressure, NG = not given, SD = standard deviation, TIA = transient ischemic attack, UT = usual treatment.
Primary outcomes
Feasibility of CPAP therapy
CPAP adherence
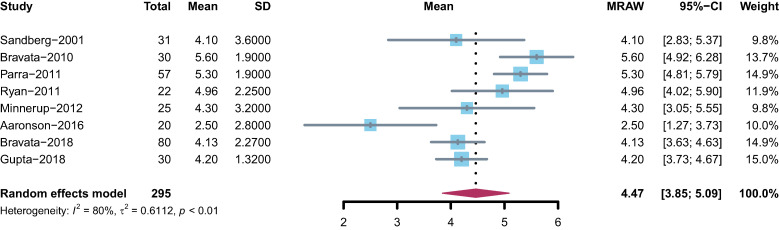
Seven RCTs19,21–24,26,27 reported a mean CPAP use of > 4 hours/night, and 1 study25 reported a mean CPAP use of < 4 hours/night, resulting in a combined mean CPAP use of 4.47 hours/night (95% CI: 3.85–5.09); however, there was considerable heterogeneity (I2 = 80%, P < .01) across studies (Figure 2).
Figure 2. Forest plot representing the adherence to CPAP treatment in hours per night.
CI = confidence interval, CPAP = continuous positive airway pressure, MRAW = untransformed/raw mean.
Dropout rate
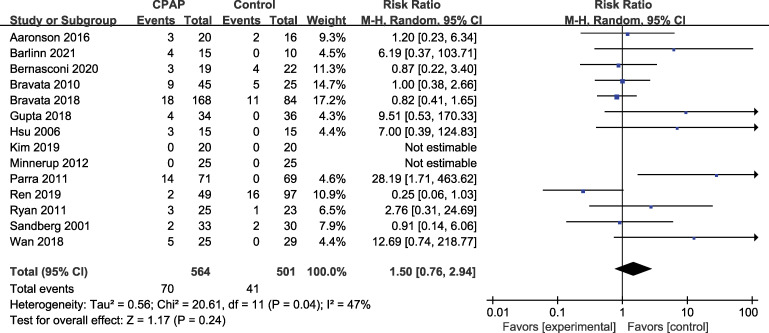
The dropout rate analysis indicated that 70 participants (70/564, 12.41%) dropped out of the intervention group compared to 41 participants (41/501, 8.18%) in the control group, with no significant difference (P = .24). The risk ratio was 1.50 (95% CI: 0.76–2.94) with low heterogeneity (I2 = 47%) (Figure 3).
Figure 3. Forest plot representing the probability of dropping out.
CI = confidence interval, CPAP = continuous positive airway pressure, M-H = mantal-haenszl.
Neurological function
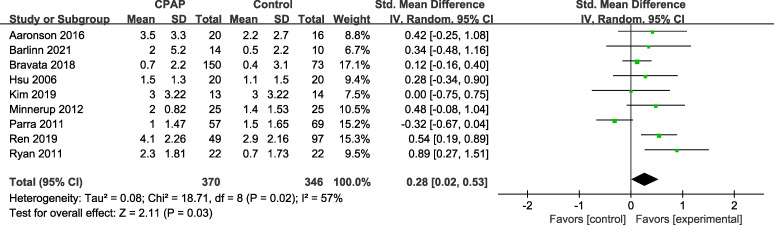
Nine studies evaluated the effect of CPAP on neurological function, 7 studies20,24–26,29,30,32 used the National Institute of Health Stroke Scale, and 2 studies22,23 used the Canadian Neurological Scale. The combined analysis of the neurofunctional scales showed neurofunctional improvement after CPAP intervention (SMD = 0.28; 95% CI: 0.02–0.53), but with substantial heterogeneity (I2 = 57%, P = .03) across the studies (Figure 4).
Figure 4. Forest plot representing SMD and 95% CI of neurological function.
CI = confidence interval, CPAP = continuous positive airway pressure, IV = inverse variance, SMD = standardized mean difference, Std. = standardized.
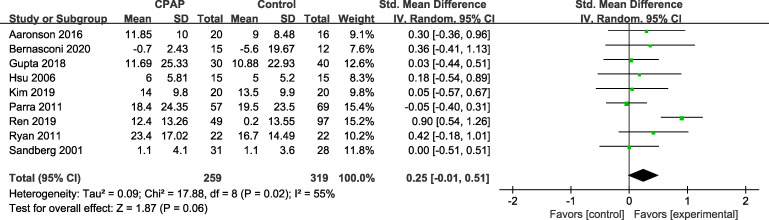
Functional status
Seven studies19,20,22,27,29–31 assessed functional status with the Barthel index, and the 2 remaining studies used the Functional Independence Measure23 and physical function subscale (mobility and self-care) in the Utrecht Scale for Evaluation of Rehabilitation.25 As shown in Figure 5, improvements in functional status with CPAP therapy in patients with stroke and SA were not significantly different from those in controls (SMD = 0.25; 95% CI: −0.01 to 0.51) and showed substantial heterogeneity (I2 = 55%, P = .06).
Figure 5. Forest plot representing SMD and 95% CI of functional status.
CI = confidence interval, CPAP = continuous positive airway pressure, IV = inverse variance, SMD = standardized mean difference, Std. = standardized.
Secondary outcomes
Recurrent vascular events
Six RCTs21,22,26,27,31,32 reported recurrent vascular events. However, there was no significant difference between the number of affected individuals in the intervention group (28/352, 7.95%) and the control group (28/246, 11.38%) (Figure S1 (10.5MB, pdf) in the supplemental material). The risk ratio was 0.70 (95% CI: 0.37–1.32; P = .27) with low heterogeneity (I2 = 14%).
Cognitive function
Four studies19,27,29,30 used the Mini-Mental State Examination to assess cognitive function, 1 study28 used the Montreal Cognitive Assessment, and 1 study20 used the Addenbrooke’s Cognitive Examination. After meta-analysis (Figure S2 (10.5MB, pdf) ), the data showed considerable heterogeneity (I2 = 91%, P = .35), and CPAP intervention had no effect on cognitive function improvement in patients with stroke and SA (SMD = 0.34; 95% CI: −0.38 to 1.07).
Depression assessments
Six studies assessed the improvement of mood disorders with CPAP therapy, 2 studies20,25 used the Hospital Anxiety and Depression Scale–Depression subscale, and the remaining studies used the Montgomery-Asberg Depression Rating Scale,19 Beck Depression Inventory-1,23 Patient Health Questionnaire-9,28 and Hamilton Rating Scale for Depression.30 There was no significant effect of CPAP on depressive symptoms (SMD = −0.42; 95% CI: −0.86 to 0.03) and with a substantial heterogeneity (I2 = 74%, P = .07) (Figure S3 (10.5MB, pdf) ).
Sleepiness
Four studies23,27–29 used the Epworth Sleepiness Scale to assess sleepiness, and 2 studies20,25 used the Stanford Sleepiness Scale. The result (Figure S4 (10.5MB, pdf) ) showed that the improvement in sleepiness between the CPAP intervention and control groups was not significant (SMD = −0.49; 95% CI: −1.06 to 0.08), and with considerable heterogeneity (I2 = 80%, P = 0.09).
Reduction in AHI
Following the intervention, 3 studies23,29,30 reported a reduction in AHI in both the intervention and control groups, while 2 studies24,28 reported a reduction in AHI only in the intervention group. Therefore, we analyzed the reduction in AHI in the intervention group in these studies. The preintervention to postintervention analysis of 5 studies showed an estimate of mean change in AHI by −20.59 events/h (95% CI: −26.30 to −14.87; P < .01) with an I2 = 73% (Figure S5 (10.5MB, pdf) ).
Adverse events
Details of adverse events during CPAP intervention were reported in 2 RCTs.21,32 No patient reported serious adverse events, and 9 patients in these studies experienced nonserious adverse events during CPAP therapy (9/54, 16.67%). Nonserious adverse events related to the intervention included local complaints (ie, local irritation of skin/mucosa and mucosal dryness) and symptoms such as sneezing and nasal irritation caused by the face mask.
Subgroup analyses
To explore the potential source of heterogeneity among studies, we conducted a series of subgroup analyses of the primary outcomes (neurological function and functional status) for different variables of studies to examine the possible sources of the heterogeneity; for example, initiation time of CPAP since stroke (within 7 days or after 7 days), exclusion of previously diagnosed SA (yes or no), form of sleep apnea (definite OSA or unclassified SA), CPAP treatment duration (≤ 6 mo or > 6 mo), and sample size of included studies (≤ 50 participants or > 50 participants). The results of all the subgroup analyses are presented in Table 2; the subgroup analyses of neurological function and functional status are shown in Figure S6 (10.5MB, pdf) and Figure S7 (10.5MB, pdf) , respectively.
Table 2.
Results of subgroup analysis on neurological function/functional status.
| Characteristics | Number of Contrast Groups | SMD | Heterogeneity | P a | |||
|---|---|---|---|---|---|---|---|
| SMD | 95% CI | P | I2 (%) | ||||
| Initiation time of CPAP since stroke | |||||||
| Neurological function | Within 7 days | 4 | 0.09 | –0.26 to 0.43 | .62 | 58 | .06 |
| After 7 days | 5 | 0.48 | 0.24 to 0.72 | <.0001 | 0 | ||
| Functional status | Within 7 days | 1 | −0.05 | −0.40 to 0.31 | .80 | NA | .12 |
| After 7 days | 8 | 0.31 | 0.03 to 0.59 | .03 | 49 | ||
| Exclusion of previously diagnosed sleep apnea | |||||||
| Neurological function | Yes | 6 | 0.28 | −0.06 to 0.61 | .10 | 71 | .89 |
| No | 3 | 0.32 | −0.08 to 0.71 | .12 | 0 | ||
| Functional status | Yes | 5 | 0.34 | −0.09 to 0.76 | .12 | 73 | .35 |
| No | 4 | 0.09 | −0.20 to 0.38 | .53 | 0 | ||
| Type of sleep apnea | |||||||
| Neurological function | Definite OSA | 5 | 0.34 | −0.12 to 0.79 | .15 | 76 | .56 |
| Unclassified sleep apnea | 4 | 0.18 | −0.04 to 0.41 | .11 | 0 | ||
| Functional status | Definite OSA | 6 | 0.29 | −0.07 to 0.64 | .12 | 70 | .55 |
| Unclassified sleep apnea | 3 | 0.13 | −0.24 to 0.49 | .49 | 0 | ||
| CPAP treatment duration | |||||||
| Neurological function | ≤ 6 months | 6 | 0.43 | 0.16 to 0.70 | .002 | 0 | .24 |
| > 6 months | 3 | 0.12 | −0.34 to 0.57 | .62 | 83 | ||
| Functional status | ≤ 6 months | 6 | 0.14 | −0.10 to 0.37 | .25 | 0 | .46 |
| > 6 months | 3 | 0.41 | −0.27 to 1.09 | .24 | 85 | ||
| Sample size of included studies | |||||||
| Neurological function | ≤ 50 participants | 6 | 0.43 | 0.16 to 0.70 | .002 | 0 | .24 |
| > 50 participants | 3 | 0.12 | −0.34 to 0.57 | .62 | 83 | ||
| Functional status | ≤ 50 participants | 5 | 0.26 | −0.04 to 0.56 | .09 | 0 | .93 |
| > 50 participants | 4 | 0.23 | −0.26 to 0.73 | .35 | 82 | ||
P values indicate whether the difference between the effect sizes in the subgroups is significant. CI = confidence interval, CPAP = continuous positive airway pressure, NA = not applicable, OSA = obstructive sleep apnea, SMD = standardized mean difference.
There were no significant differences in terms of neurological function between the subgroups. The subgroup analysis on the initiation time of CPAP demonstrated that the group in which CPAP was started within 7 days after stroke group showed greater improvement in neurological function than the control group (SMD = 0.48; 95% CI: 0.24–0.72; P < .0001) (Figure S6A (10.5MB, pdf) ). However, the group in which CPAP was started within 7 days after stroke did not show greater improvement in neurological function than the control group. In relation to neurological function, regardless of whether previously diagnosed SA was excluded or the form of SA, there were no significant differences between the CPAP and control groups (Figure S6B (10.5MB, pdf) , Figure S6C (10.5MB, pdf) ). CPAP treatment was more beneficial compared with usual treatment for the restoration of neurological function when the CPAP duration was < 6 months (SMD = 0.43; 95% CI: 0.16–0.70; P = .002) (Figure S6D (10.5MB, pdf) ). Subgroup analysis of studies with sample sizes ≤ 50 showed that there was a significant difference between the CPAP and control group (SMD = 0.43; 95% CI: 0.16–0.70; P = .002) (Figure S6E (10.5MB, pdf) ).
There was no significant difference in the functional status between the subgroups (Figure S7 (10.5MB, pdf) ). In subgroup analyses of functional status, CPAP started 7 days after stroke showed a significant difference when compared with the control group (SMD = 0.31; 95% CI: 0.03–0.59; P = .03) (Figure S7A (10.5MB, pdf) ). However, compared with the control group, CPAP started within 7 days since stroke group did not show a greater improvement in the functional status.
Sensitivity analyses
Because there was substantial heterogeneity across studies for the outcomes of neurological function (I2 = 57%) and functional status (I2 = 55%), sensitivity analyses were performed for these outcomes (Figure S8 (10.5MB, pdf) ). In the sensitivity analysis of neurological function, after excluding 1 study,22 the effect size was higher (SMD = 0.36; 95% CI: 0.17–0.54; P = .0002) (Figure S8A (10.5MB, pdf) ), with a significant change in heterogeneity (I2 = 10%). In the sensitivity analysis of the functional status, after excluding 1 study,30 the heterogeneity decreased (I2 = 0%). However, the overall results were not influenced by the individual studies (Figure S8B (10.5MB, pdf) ).
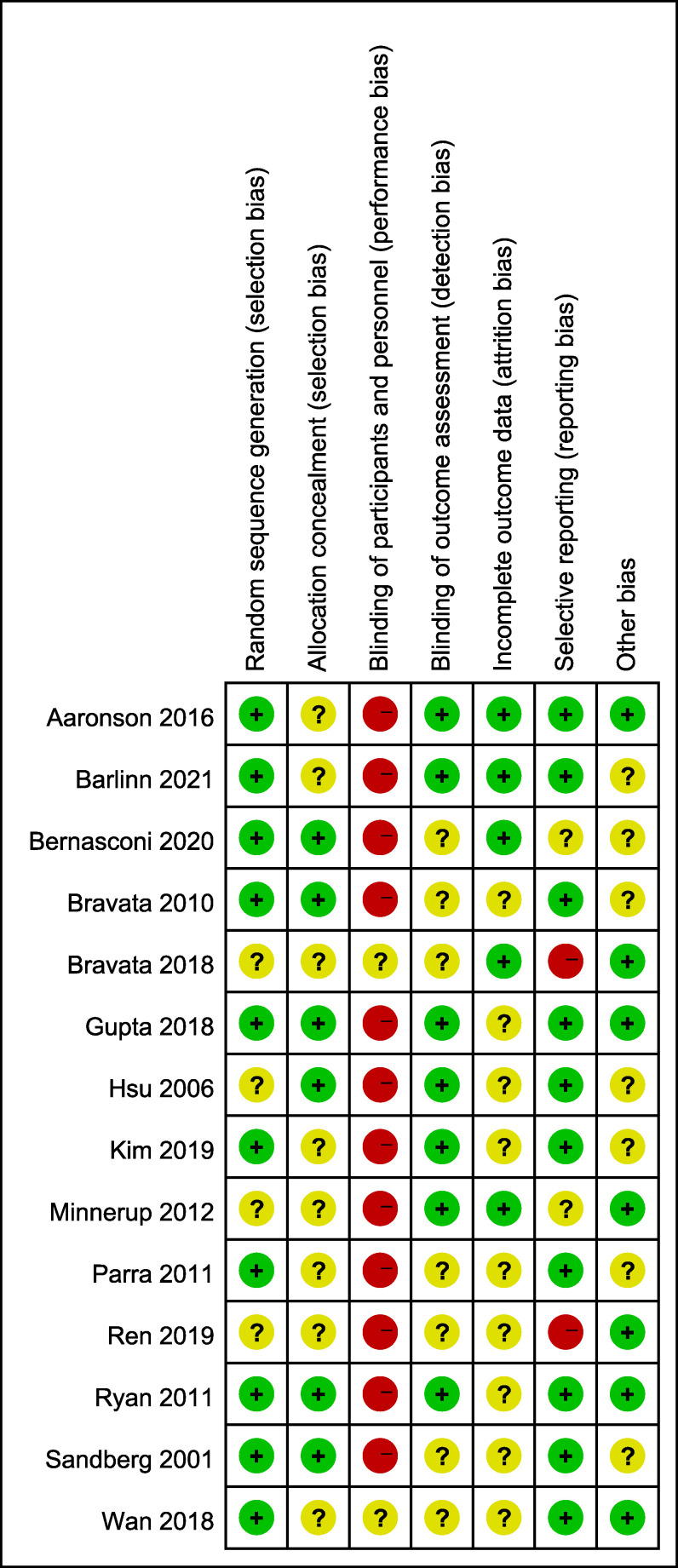
Assessment of risk of bias
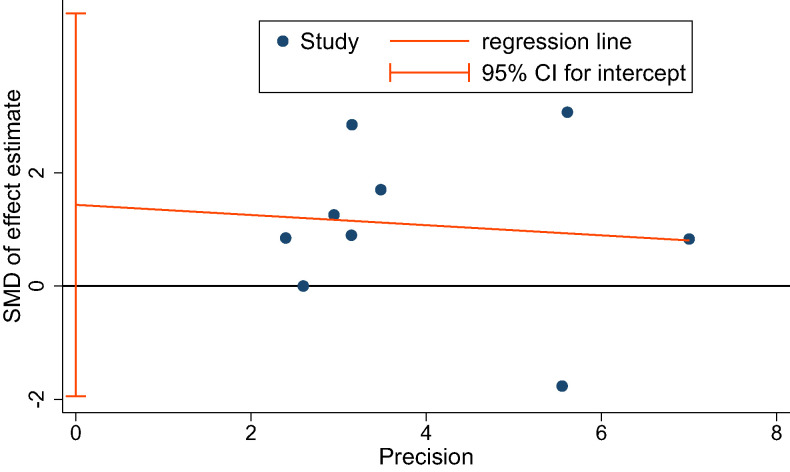
The overall quality of the studies included in this meta-analysis was moderate. Detailed results of bias assessment are presented in Figure 6 and Figure S9 (10.5MB, pdf) . A funnel plot of the primary efficacy outcome is presented in Figure S10 (10.5MB, pdf) to assess publication bias, with no significant asymmetry detectable. The Egger’s regression test also showed no significant evidence of publication bias (P = .349) (Figure 7).
Figure 6. Risk-of-bias summary: review authors’ judgments about each risk-of-bias item for each included study.
Figure 7. Egger’s publication bias plot.
CI = confidence interval, SMD = standardized mean difference.
DISCUSSION
This meta-analysis found that CPAP therapy is feasible in patients with early- and late-onset stroke complicated by SA, and CPAP may improve neurological outcomes. This study supported further research in this area. This meta-analysis included 14 RCTs that evaluated the effect of CPAP compared with that of usual treatment in patients with stroke or TIA with SA. Compared with the previous studies, our study added functional status as a primary outcome measure, and the results showed that current data are insufficient to conclude that CPAP improves functional outcomes in patients with stroke and SA. Despite a trend of lower risk for recurrent vascular events in CPAP-treated patients, the difference was not significant. Additionally, there was no improvement in cognitive function, depression, and sleepiness after CPAP intervention.
With regard to the feasibility of CPAP therapy, our result showed that the mean duration of CPAP use was 4.47 hours/night, and the adherence was acceptable. These findings are similar to those presented in a previous meta-analysis published by Brill et al.16 The dropout rate was 12.41% (70/564) in the intervention group and 8.18% (41/501) in the control group. It was higher in the experimental group but did not differ significantly from that in the control group. CPAP therapy was safe, and no serious adverse events related to the intervention were reported in any of the trials. The nonserious adverse events related to the face mask were more common in the CPAP group and mainly included local irritation of the skin or mucosa and symptoms such as sneezing and nasal irritation. The above results indicated that CPAP therapy is feasible in patients with stroke and SA. However, this result needs to be interpreted cautiously due to the high heterogeneity among the included studies.
The current analysis found that CPAP therapy in patients with stroke and SA provides a beneficial effect on neurological recovery, consistent with the results of previous meta-analyses.15,16 However, there was substantial heterogeneity across studies evaluating neurological function (I2 = 57%), and the possible sources of heterogeneity were found on the exclusion of 1 study. Reanalysis after the exclusion of the study22 reduced heterogeneity significantly. The possible sources of heterogeneity were as follows: (1) the study was low quality; (2) patients were not blinded to the intervention and blinding of outcome assessments in the study was not guaranteed; thus, the results of the study might be affected; and (3) there were significant baseline differences between the intervention and control groups.
We also conducted subgroup analyses on the outcome of neurological function to determine the sources of heterogeneity. The results indicated that there were no significant differences between all subgroups. Subgroup analysis according to the time of CPAP initiation since stroke showed that early CPAP therapy had no effect, while CPAP therapy beyond 7 days was beneficial. Although there was no statistical difference between this subgroup, the time point of receiving CPAP after stroke may be 1 source of heterogeneity. CPAP treatment can ameliorate nocturnal intermittent hypoxia and cerebrovascular hemodynamics caused by SA and promote the recovery of neurological function. Specifically, in patients with ischemic stroke, early application of CPAP can prolong the survival of the penumbra, resulting in clinical and imaging improvement. However, our findings did not show a favorable effect of CPAP intervention on the acute phase of stroke, which we speculate may be related to the length of the intervention in these included studies. In the included studies, all patients who received CPAP therapy within 7 days after stroke onset had ischemic stroke or TIA. Two of the included studies assessed neurological function at 3 days32 and 8 days,24 respectively, and did not detect an improvement in neurological function with CPAP. Another prospective observational study33 assessing noninvasive mechanical ventilation within 48 hours after stroke onset showed significant improvement in National Institute of Health Stroke Scale scores in the subacute (2 wk) and chronic (2 mo) phases. Future studies with a longer duration of intervention in the acute phase may be needed to verify the effectiveness of early CPAP intervention for acute patients with stroke and SA.
The subgroup analysis based on the CPAP treatment duration showed significantly better neurological improvement in the intervention group with an intervention time ≤ 6 months than that in the control group. However, this was not observed in the intervention group with an intervention time > 6 months. In the subgroup with treatment duration > 6 months, the mean treatment duration was more than 1 year. Generally, stroke patients recover from neurological deficits in the late stage, and their condition stabilizes during this period. Johnson et al34 reported that using CPAP during inpatient rehabilitation after stroke in patients with SA appears to accelerate recovery in the short term, but the long-term benefits remain unclear. This may suggest that CPAP intervention for ≤ 6 months can improve neurological function in patients with stroke and SA, but extending the treatment time does not increase the treatment effect.
Our study found that CPAP intervention did not improve the functional status of patients. This may be because scales to assess functional status have certain limitations. The partial assessment items require patients to have a high degree of completion, with a limited assessment of slight functional improvement. Given that there was substantial heterogeneity across studies on the outcomes of functional status (I2 = 55%), the sensitivity analysis was carried out. After excluding 1 study,30 the heterogeneity decreased (I2 = 0%). The possible sources of heterogeneity in this study were as follows: (1) the study had low quality; (2) there were performance and detection biases in this study because blinding was not used between researchers and participants, and blinding was not mentioned in measuring outcome measures; and (3) the number of participants in the treatment and intervention groups differed significantly, and the study used the last measured data to replace partially missing data, which may have influenced the results.
Subgroup analyses for functional status as an outcome measure also showed that there was no difference between subgroups. However, subgroup analysis based on CPAP initiation time since stroke onset showed that early CPAP use had no effect, while CPAP initiation beyond 7 days of stroke onset is beneficial. Functional status is an important outcome measure in stroke patients as it can, to a certain extent, indicate the motor function of patients, Further, it follows the direction of neurological recovery in stroke patients. However, motor function recovery is later than neurological function recovery in stroke patients. An RCT conducted task-specific motor intervention in stroke patients and found that the optimal period for motor recovery ranges from 60 days to 90 days after stroke, with lesser effects ≤ 30 days and no effect at 6 months or later after stroke.35 These data provide a time window to further explore whether CPAP intervention can improve motor function and functional status of stroke patients.
In our study, although the incidence of recurrent vascular events was lower in the CPAP group than in the control group (7.95% [28/352] vs 11.38% [28/246]), the difference was not significant. Current studies have shown improved outcomes in post-stroke patients with OSA treated with CPAP. However, data on risk reduction of subsequent ischemic stroke are rare.36 Boulos et al37 also reported that it remains unclear whether CPAP (or other treatment modalities for SA) affects vascular outcomes (eg, recurrent stroke and mortality in post-stroke SA) owing to the limited quality of current articles. Therefore, well-designed clinical trials of CPAP or other modalities to treat post-stroke SA are needed.
Our study found no significant difference in cognitive function between the CPAP intervention group and the control group. However, the meta-analysis of cognitive function was highly heterogeneous (I2 = 91%); thus, this result should be interpreted with caution. Given that cognitive function was analyzed using cognitive function scales, the data reflected overall assessments of cognitive function. Total scale scores are not sufficiently accurate to assess improvements in cognitive function. Three included studies25,27,28 assessed domains of refined cognitive function and concluded that attention and delayed recall were improved. However, the methods used for assessment were inconsistent and difficult to analyze uniformly. One study29 categorized the cognitive domains in the Mini-Mental State Examination and concluded that there was improvement in attention and calculation. However, this study had low sensitivity due to the relatively simple assessment items of the Mini-Mental State Examination. Therefore, it is necessary to further refine assessment methods in cognitive domains and use uniform criteria to more accurately assess cognitive function. For depression as an outcome measure, we found no improvement in depressive symptoms, with no significant difference between the CPAP intervention group and the control group.
With regard to sleepiness, there was no significant difference between the CPAP intervention and control groups. However, this should also be interpreted cautiously because of the large heterogeneity of the outcome analysis. The heterogeneity may be derived from varied criteria for AHI in the included studies. AHI can reflect the severity of SA. Nocturnal SA can increase the risk of excessive daytime sleepiness. The sleepiness score also can reflect, to some extent, the nocturnal sleep apnea index.38 In the current study, CPAP reduced AHI by 20.55 events/h, but there was considerable heterogeneity in the studies, which possibly resulted from the differences in the criteria for AHI in the included studies.
Limitations
The limitations of this study are as follows. First, risk of bias was considerably moderate in all included studies. Different study designs may be used in the future to improve the quality of the articles. Moreover, some of the included RCTs had a small sample size; thus, the test power was low. The second limitation is that the study population had varying stroke types, stroke locations, and AHI values, and it was possible that some patients with severe stroke and symptomatic SA were excluded, resulting in the higher CPAP adherence. It is likely that the studies required a larger sample size and expanded the application of CPAP. Although these may have increased the heterogeneity of the studies, the results have also become more generalizable and reliable. Further, this allowed us to perform more subgroup analyses to clarify whether the intervention applies to more patients with different conditions or only to a specific population. The last limitation is that, in the outcome analyses, studies had large heterogeneity; thus, the results need to be interpreted with caution.
CONCLUSIONS
CPAP therapy is a feasible modality in patients with stroke associated with SA and improves neurological outcomes in these patients. However, this finding should be interpreted with caution because of the substantial heterogeneity of trials and low literature quality. This improved outcome is more obvious with short-term intervention (≤ 6 mo), and prolonging the intervention time did not increase the therapeutic effect. This study did not detect an improvement in neurological function with CPAP in the acute phase of stroke. Further, there were no significant differences in functional status and recurrence of vascular events between patients who did and did not receive CPAP treatment. Current data are insufficient to draw conclusions on the beneficial effectiveness of CPAP on cognitive function, sleepiness, and depression in these patients. Additional high-quality RCTs are needed to clarify the role of CPAP in patients with stroke and SA.
DISCLOSURE STATEMENT
All authors read and approved the final version of the manuscript. Work for this study was performed at The First Affiliated Hospital of Chongqing Medical University. This study was funded by The Chongqing Municipal Health Commission Project (grant no. 2018jstg007), the National Natural Science Foundation of China (grant no. 82002396), and the In-Hospital Cultivation Fund of the First Affiliated Hospital of Chongqing Medical University (grant no. PYJJ2021-07). The authors report no conflicts of interest.
ACKNOWLEDGMENTS
Author contributions: S.F. conceived the study. S.F. and L.Y. drafted the manuscript. Y.L., X.P., and H.Y. assisted in the design and revision. S.F. and L.Y. designed the statistical analysis. L.Y. and H.Y. are the guarantors.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- CI
confidence interval
- CPAP
continuous positive airway pressure
- OSA
obstructive sleep apnea
- RCT
randomized controlled trial
- SA
sleep apnea
- SMD
standardized mean difference
- TIA
transient ischemic attack
REFERENCES
- 1. Yaggi HK , Concato J , Kernan WN , Lichtman JH , Brass LM , Mohsenin V . Obstructive sleep apnea as a risk factor for stroke and death . N Engl J Med. 2005. ; 353 ( 19 ): 2034 – 2041 . [DOI] [PubMed] [Google Scholar]
- 2. Seiler A , Camilo M , Korostovtseva L , et al . Prevalence of sleep-disordered breathing after stroke and TIA: a meta-analysis . Neurology. 2019. ; 92 ( 7 ): e648 – e654 . [DOI] [PubMed] [Google Scholar]
- 3. Liu X , Lam DC , Chan KPF , Chan HY , Ip MS , Lau KK . Prevalence and determinants of sleep apnea in patients with stroke: a meta-analysis . J Stroke Cerebrovasc Dis. 2021. ; 30 ( 12 ): 106129 . [DOI] [PubMed] [Google Scholar]
- 4. Tanayapong P , Kuna ST . Sleep disordered breathing as a cause and consequence of stroke: a review of pathophysiological and clinical relationships . Sleep Med Rev. 2021. ; 59 : 101499 . [DOI] [PubMed] [Google Scholar]
- 5. Iranzo A , Santamaría J , Berenguer J , Sánchez M , Chamorro A . Prevalence and clinical importance of sleep apnea in the first night after cerebral infarction . Neurology. 2002. ; 58 ( 6 ): 911 – 916 . [DOI] [PubMed] [Google Scholar]
- 6. Kaneko Y , Hajek VE , Zivanovic V , Raboud J , Bradley TD . Relationship of sleep apnea to functional capacity and length of hospitalization following stroke . Sleep. 2003. ; 26 ( 3 ): 293 – 297 . [DOI] [PubMed] [Google Scholar]
- 7. Xie W , Zheng F , Song X . Obstructive sleep apnea and serious adverse outcomes in patients with cardiovascular or cerebrovascular disease: a PRISMA-compliant systematic review and meta-analysis . Medicine (Baltimore). 2014. ; 93 ( 29 ): e336 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Birkbak J , Clark AJ , Rod NH . The effect of sleep disordered breathing on the outcome of stroke and transient ischemic attack: a systematic review . J Clin Sleep Med. 2014. ; 10 ( 1 ): 103 – 108 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ahn SH , Kim JH , Kim DU , Choo IS , Lee HJ , Kim HW . Interaction between sleep-disordered breathing and acute ischemic stroke . J Clin Neurol. 2013. ; 9 ( 1 ): 9 – 13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davis AP , Billings ME , Longstreth WT Jr , Khot SP . Early diagnosis and treatment of obstructive sleep apnea after stroke: are we neglecting a modifiable stroke risk factor? Neurol Clin Pract. 2013. ; 3 ( 3 ): 192 – 201 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sharma S , Srijithesh PR . Sleeping over a sleep disorder—awareness of obstructive sleep apnoea as a modifiable risk factor for hypertension and stroke: a survey among health care professionals and medical students . Ann Indian Acad Neurol. 2013. ; 16 ( 2 ): 151 – 153 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown DL , Jiang X , Li C , et al . Sleep apnea screening is uncommon after stroke . Sleep Med. 2019. ; 59 : 90 – 93 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kernan WN , Ovbiagele B , Black HR , et al. American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Peripheral Vascular Disease . Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association . Stroke. 2014. ; 45 ( 7 ): 2160 – 2236 . [DOI] [PubMed] [Google Scholar]
- 14. Patil SP , Ayappa IA , Caples SM , Kimoff RJ , Patel SR , Harrod CG . Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine Systematic Review, Meta-Analysis, and GRADE Assessment . J Clin Sleep Med. 2019. ; 15 ( 2 ): 301 – 334 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tsivgoulis G , Alexandrov AV , Katsanos AH , et al . Noninvasive ventilatory correction in patients with acute ischemic stroke: a systematic review and meta-analysis . Stroke. 2017. ; 48 ( 8 ): 2285 – 2288 . [DOI] [PubMed] [Google Scholar]
- 16. Brill A-K , Horvath T , Seiler A , et al . CPAP as treatment of sleep apnea after stroke: a meta-analysis of randomized trials . Neurology. 2018. ; 90 ( 14 ): e1222 – e1230 . [DOI] [PubMed] [Google Scholar]
- 17. Moher D , Liberati A , Tetzlaff J , Altman DG ; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement . Open Med. 2009. ; 3 ( 3 ): e123 – e130 . [PMC free article] [PubMed] [Google Scholar]
- 18. Higgins JPT, Green S , eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available: www.handbook.cochrane.org . [Google Scholar]
- 19. Sandberg O , Franklin KA , Bucht G , Eriksson S , Gustafson Y . Nasal continuous positive airway pressure in stroke patients with sleep apnoea: a randomized treatment study . Eur Respir J. 2001. ; 18 ( 4 ): 630 – 634 . [DOI] [PubMed] [Google Scholar]
- 20. Hsu CY , Vennelle M , Li HY , Engleman HM , Dennis MS , Douglas NJ . Sleep-disordered breathing after stroke: a randomised controlled trial of continuous positive airway pressure . J Neurol Neurosurg Psychiatry. 2006. ; 77 ( 10 ): 1143 – 1149 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bravata DM , Concato J , Fried T , et al . Auto-titrating continuous positive airway pressure for patients with acute transient ischemic attack: a randomized feasibility trial . Stroke. 2010. ; 41 ( 7 ): 1464 – 1470 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Parra O , Sánchez-Armengol A , Bonnin M , et al . Early treatment of obstructive apnoea and stroke outcome: a randomised controlled trial . Eur Respir J. 2011. ; 37 ( 5 ): 1128 – 1136 . [DOI] [PubMed] [Google Scholar]
- 23. Ryan CM , Bayley M , Green R , Murray BJ , Bradley TD . Influence of continuous positive airway pressure on outcomes of rehabilitation in stroke patients with obstructive sleep apnea . Stroke. 2011. ; 42 ( 4 ): 1062 – 1067 . [DOI] [PubMed] [Google Scholar]
- 24. Minnerup J , Ritter MA , Wersching H , et al . Continuous positive airway pressure ventilation for acute ischemic stroke: a randomized feasibility study . Stroke. 2012. ; 43 ( 4 ): 1137 – 1139 . [DOI] [PubMed] [Google Scholar]
- 25. Aaronson JA , Hofman WF , van Bennekom CA , et al . Effects of continuous positive airway pressure on cognitive and functional outcome of stroke patients with obstructive sleep apnea: a randomized controlled trial . J Clin Sleep Med. 2016. ; 12 ( 4 ): 533 – 541 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bravata DM , Sico J , Vaz Fragoso CA , et al . Diagnosing and treating sleep apnea in patients with acute cerebrovascular disease . J Am Heart Assoc. 2018. ; 7 ( 16 ): e008841 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gupta A , Shukla G , Afsar M , et al . Role of positive airway pressure therapy for obstructive sleep apnea in patients with stroke: a randomized controlled trial . J Clin Sleep Med. 2018. ; 14 ( 4 ): 511 – 521 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wan Y , Zhao F , Liu L , Cui L , Qiu Z , Xue R . Influence of continuous positive airway pressure on sleep status, neuropsychological characteristics in patients with stroke combined with obstructive sleep apnea syndrome . Chin J Neurol. 2018. ; 51 ( 4 ): 256 – 262 . [Google Scholar]
- 29. Kim H , Im S , Park JI , Kim Y , Sohn MK , Jee S . Improvement of cognitive function after continuous positive airway pressure treatment for subacute stroke patients with obstructive sleep apnea: a randomized controlled trial . Brain Sci. 2019. ; 9 ( 10 ): 252 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ren L , Wang K , Shen H , Xu Y , Wang J , Chen R . Effects of continuous positive airway pressure (CPAP) therapy on neurological and functional rehabilitation in basal ganglia stroke patients with obstructive sleep apnea: a prospective multicenter study . Medicine (Baltimore). 2019. ; 98 ( 28 ): e16344 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bernasconi C , Ott SR , Fanfulla F , et al . SAS CARE 2—a randomized study of CPAP in patients with obstructive sleep disordered breathing following ischemic stroke or transient ischemic attack . Sleep Med. 2020. ; 2 : 10027 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barlinn K , Jakubicek S , Siepmann T , et al . Autotitrating bilevel positive airway pressure in large vessel steno-occlusive stroke patients with suspected sleep apnea: a multicenter randomized controlled study . Front Neurol. 2021. ; 12 : 667494 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Benbir G , Karadeniz D . A pilot study of the effects of non-invasive mechanical ventilation on the prognosis of ischemic cerebrovascular events in patients with obstructive sleep apnea syndrome . Neurol Sci. 2012. ; 33 ( 4 ): 811 – 818 . [DOI] [PubMed] [Google Scholar]
- 34. Johnson BP , Shipper AG , Westlake KP . Systematic review investigating the effects of nonpharmacological interventions during sleep to enhance physical rehabilitation outcomes in people with neurological diagnoses . Neurorehabil Neural Repair. 2019. ; 33 ( 5 ): 345 – 354 . [DOI] [PubMed] [Google Scholar]
- 35. Dromerick AW , Geed S , Barth J , et al . Critical Period After Stroke Study (CPASS): a phase II clinical trial testing an optimal time for motor recovery after stroke in humans . Proc Natl Acad Sci USA. 2021. ; 118 ( 39 ): e2026676118 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Parasram M , Segal AZ . Sleep disorders and stroke: does treatment of obstructive sleep apnea decrease risk of ischemic stroke? Curr Treat Options Neurol. 2019. ; 21 ( 7 ): 29 . [DOI] [PubMed] [Google Scholar]
- 37. Boulos MI , Dharmakulaseelan L , Brown DL , Swartz RH . Trials in sleep apnea and stroke: learning from the past to direct future approaches . Stroke. 2021. ; 52 ( 1 ): 366 – 372 . [DOI] [PubMed] [Google Scholar]
- 38. Guo Q , Song WD , Li W , et al . Weighted Epworth Sleepiness Scale predicted the apnea-hypopnea index better . Respir Res. 2020. ; 21 ( 1 ): 147 . [DOI] [PMC free article] [PubMed] [Google Scholar]