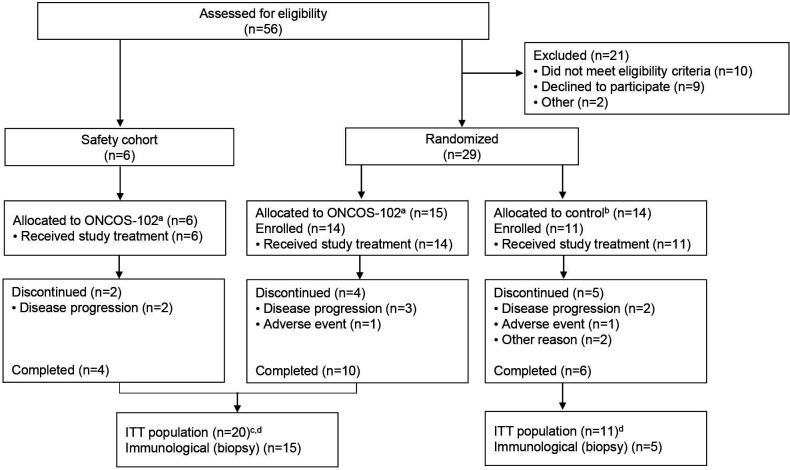
Figure 2.
Patient disposition. aAllocated to ONCOS-102 (3×1011 virus particles in 2.5 mL) with pemetrexed (500 mg/m2) in combination with cisplatin (75 mg/m2) or carboplatin (AUC 5) in 21-day cycles. bRandomized to pemetrexed (500 mg/m2) in combination with cisplatin (75 mg/m2) or carboplatin (AUC 5) in 21-day cycles. cData were pooled from safety cohort and ONCOS-102 randomized cohort. dAs all enrolled patients received study treatment, the ITT population was used to summarize efficacy and safety outcomes. AUC, area under the concentration–time curve; ITT, intention to treat.