Abstract
Background
Prosthetic valve thrombosis (PVT) is a severe complication of mechanical valve replacement. Simultaneous thrombosis of multiple prosthetic valves is rare and is associated with worse outcomes. Treatment options include anticoagulation, thrombolysis, and redo operative valve replacement, with rare reports of adjunctive balloon valvuloplasty. There is limited evidence to guide therapeutic selection, specifically dosing, timing, and duration of thrombolysis. The following case series highlights the importance of successful thrombolytic management in dual PVT with high bleeding risk defined as a coagulopathy with an elevated international normalized ratio greater than 3 and New York Heart Association (NYHA) Class III and IV heart failure.
Case summary
We describe two patients with concomitant aortic and mitral PVT. Both patients presented in NYHA Class III and IV heart failure with different challenges to surgical treatments including high bleeding risk from coagulopathy and history of multiple prior sternotomies. After multi-disciplinary discussions, both patients underwent a combination of low-dose, slow, or ultra-slow infusion of tissue plasminogen activator, with a resolution of their dual PVT seen on cine-fluoroscopy imaging as freely moving mechanical leaflets and improvement of heart failure symptoms back to baseline NYHA Class II or lower.
Discussion
Prosthetic valve thrombosis is a complex medical condition requiring a multi-disciplinary team to evaluate the best course of treatment. A trial of pharmacologic thrombolysis is often the first attempted treatment for obstructive PVT, although surgery is recommended for patients with NYHA IV symptoms, or with contraindications to thrombolysis, including high bleeding risk. However, in patients with high bleeding risk and NYHA Class III and IV heart failure, especially with surgical contraindications, low-dose thrombolytics, with slow or ultra-slow infusions, may still be safe and effective treatment strategies for multi-valve PVT. Further research is needed to guide thrombolysis in multi-valve PVT.
Keywords: Thrombosis, Thrombolytic therapy, Heart valve prosthesis, Valvular heart Disease, Case reports
Learning points.
Patients with prosthetic multi-valve thrombosis can present with unique conditions that preclude surgical treatment, requiring a multi-disciplinary approach for optimal medical management.
Dual prosthetic valve thrombosis can be safely treated with low-dose, slow, or ultra-slow infusion of tissue plasminogen activator (tPA).
Safe baseline international normalized ratio (INR) before administration of thrombolytics has not been established. Low-dose, slow, or ultra-slow infusion of tPA may be a potential treatment for patients with elevated INR levels above 3.0, but more data are needed before extrapolating to a larger patient population.
Introduction
Prosthetic valve thrombosis (PVT) is a severe complication of mechanical valve replacement. The incidence rate of PVT is 0.1–6% in mechanical left-sided valves,1,2 and associated mortality can range from 4 to 17.5% when there is a delay in treatment or haemodynamic instability in isolated mechanical left-sided valves.2–5 Therapeutic options mainly include anticoagulation, thrombolysis, and redo operative valve replacement, with rare reports of adjunctive balloon valvuloplasty. Despite the complexity, there is limited evidence to guide therapeutic selection, notably including the candidacy, timing, dosage, and duration of thrombolytics. Furthermore, simultaneous thrombosis of multiple prosthetic valves is rare, accounting for 1–7.5% of patients in larger trials,2,3,6–8 and may be associated with worse outcomes. We report two cases of high-risk simultaneous mechanical mitral and aortic valve thrombosis treated successfully with thrombolysis. The following case series highlights the importance of successful thrombolytic management in dual PVT with coagulopathy defined as an international normalized ratio (INR) > 3.0, recent ischaemic stroke with haemorrhagic conversion, and/or New York Heart Association (NYHA) Class III and IV heart failure. Patient consent was obtained in accordance with COPE guidelines.
Summary figure
| Case Number | Time point | Event |
|---|---|---|
| 1 | Day 0 (presentation) | Presentation Shortness of breath for 3 weeks |
| Day 1 (initial assessment and management) | Labs Revealed sub-therapeutic INR of 1.4. Treatment IV diuresis and trans-thoracic echocardiogram ordered | |
| Day 2 (management) | Imaging Trans-thoracic echocardiogram revealed abnormal prosthetic AV and mitral valve gradients with regurgitation. Management Sent to the cardiovascular intensive care unit. Heparin drip initiated with anti-Xa goal 0.3–0.7 U/mL and/or aPTT goal 1.5–2.5 times the control value in seconds. | |
| Day 3 (management) | Discussion Multi-disciplinary meeting between cardiothoracic surgery, interventional cardiology, cardiac critical care, and neurology for surgical risk and whether tPA can be trialled in recent stroke. Management Initiation of tPA 25 mg over 25 h, followed by heparin drip. | |
| Day 4 (management) | Management Persistent valve obstruction noted on echocardiogram. Heparin drip stopped and 2nd dose of tPA 25 mg over 25 h followed by heparin drip. | |
| Day 5 (management) | Management Persistent valve obstruction noted on echocardiogram. Heparin drip stopped and 3rd dose of tPA 25 mg over 25 h followed by heparin drip. | |
| Day 6 (management) | Imaging Cardiac computed tomography showed improvement in prosthetic leaflet mobility of both the aortic and mitral valves suggesting resolution of the dual PVT. One day later, a follow-up trans-thoracic echocardiogram noted similar improvement in the gradient across the mechanical valves. She was then transitioned to warfarin with a goal INR of 2.5–3.5. | |
| Day 7 (management) | Imaging Trans-thoracic echocardiogram noted similar improvement in the gradient across the mechanical valves. Management Transitioned to warfarin with a goal INR of 2.5–3.5. | |
| Day 12 (management) | Discharged on warfarin with therapeutic INR 2.7. | |
| 5 months later (follow-up) | Re-presented with acute dyspnoea and heart failure due to repeat thrombosis of aortic valve resulting in demise. | |
| 2 | Day 0 (presentation) | Presentation Dyspnoea at rest and decreased exercise tolerance. |
| Day 1 (initial assessment and management) | Imaging A trans-thoracic echocardiogram showed right ventricular dysfunction, elevated mitral trans-valvular gradient, and moderate aortic regurgitation. Right heart catheterization showed elevated right and left-sided filling pressures with low cardiac output. Cine-fluoroscopy showed severely reduced leaflet mobility of the mechanical mitral and aortic valve leaflets. Labs revealed liver and kidney injury, lactic acidosis, and supra-therapeutic INR of 3.5. Discussion Multi-disciplinary meeting between cardiothoracic surgery, interventional cardiology, and cardiac critical care determined surgical risk to be prohibitive given the three prior sternotomies in addition to coagulopathy and ongoing cardiogenic shock. Treatment She was initially treated with a slow infusion of tPA 25 mg over 6 h considering her competing risks from unstable haemodynamics and coagulopathy. She also received intravenous Vitamin K 5 mg as her INR uptrended to 6.9. | |
| Days 2–4 (management) | Improvement in haemodynamics with resolution of shock. Cine-fluoroscopy showed persistently fixed mitral and aortic valve leaflets. Treatment Two additional tPA low-dose ultra-slow infusions of 25 mg over 25 h each with serial echocardiograms and interval intravenous heparin, once INR was sub-therapeutic on Day 3, were administered. | |
| Day 5 (management) | Repeat computed tomography showed freely moving aortic leaflets and persistently closed singular mitral valve leaflet. | |
| Day 6 (management) | Discharged on therapeutic warfarin with goal INR 2.5–3.5. | |
| 2 weeks later (follow-up) | Fluoroscopy at outpatient follow-up 2 weeks later showed continued immobile mitral valve leaflet, and balloon valvuloplasty was electively planned. | |
| 2 weeks later (follow-up) | Repeat fluoroscopy 2 additional weeks later showed freely moving mitral valve leaflets. |
Patient 1
A 52-year-old woman presented with dyspnoea with minimal exertion and intermittent chest pain. She had a history of severe rheumatic mitral and aortic stenosis, with mechanical mitral valve (31 mm Carbomedics, Corcym, Austin, Texas, USA) replacement 4 years prior, with a subsequent mechanical aortic valve (21 mm St. Jude, St. Jude, Saint Paul, Minnesota, USA) replacement and tricuspid valve repair (30 mm Tri Adams ring, Medtronic, Minneapolis, Minnesota, USA) 3 years prior. She had isolated PVT of her mitral valve 3.5 years before her presentation associated with temporary anticoagulation cessation that resolved with thrombolysis. She also had a history of congestive heart failure with preserved ejection fraction and multiple ischaemic strokes with haemorrhagic conversion most recently 3 months prior to this presentation. Her INR was sub-therapeutic (1.4) on admission. Her HASBLED score was 4 indicating an 8.9% risk of a major bleed with a CHA2DS2VASc score of 4. A decision to start unfractionated heparin infusion was initiated due to her sub-therapeutic INR and high suspicion of a PVT. A trans-thoracic echocardiogram (TTE) showed limited leaflet motion of the mechanical mitral valve with elevated mean trans-valvular gradient (19 mmHg), moderate prosthetic aortic regurgitation, severe prosthetic aortic valve stenosis with visualized 11 × 5 mm intra-valvular thrombus and elevated mean trans-valvular gradient (74 mmHg), and moderate to severe tricuspid regurgitation (Figure 1). No mitral valve thrombus was visualized due to shadowing from the aortic valve. A TEE was considered to better evaluate the mitral valve but was not performed due to concern for the destabilizing haemodynamic consequences of sedation while performing a trans-oesophageal echocardiography. A right heart catheterization showed right atrial pressure 10 mmHg, pulmonary artery pressure 73/28 (mean 44) mmHg, pulmonary capillary wedge pressure 32 mmHg, and a cardiac index of 2.1 L/min/m2. Cine-fluoroscopy demonstrated both mechanical valves had severely reduced leaflet motion with both valves having an unmeasurable opening angle consistent with dual mitral and aortic PVT (Figure 2).
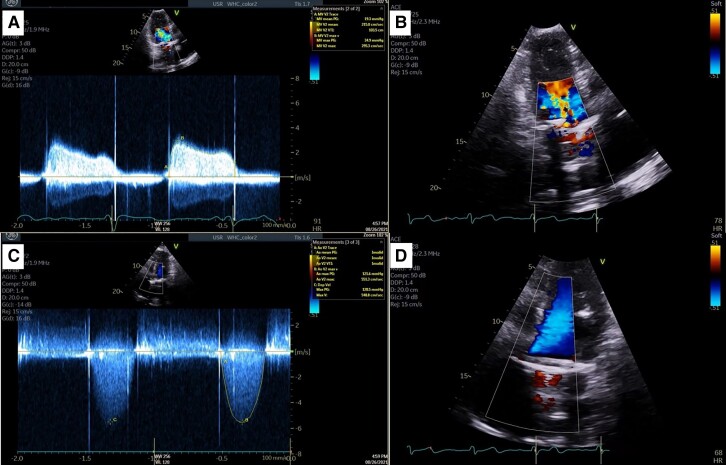
Figure 1.
Case 1. Trans-thoracic echocardiogram of mechanical mitral and aortic valves. (A) Apical two-chamber continuous wave Doppler of mitral valve inflow demonstrating an elevated pressure gradient across the mitral valve, mitral valve max velocity 2.7 m/s, mitral valve mean pressure gradient 19 mmHg at heart rate 90 b.p.m. (B) Apical two-chamber view with colour Doppler mosaic pattern indicating increased mitral inflow velocity. (C) Apical five-chamber continuous wave Doppler of aortic valve outflow demonstrating an elevated gradient across the aortic valve, aortic valve max velocity 5.5 m/s, aortic valve max PG 121 mmHg, and aortic valve mean PG 74 mmHg. (D) Apical five-chamber view of left ventricular outflow tract, left ventricular outflow tract max velocity 0.8 m/s.
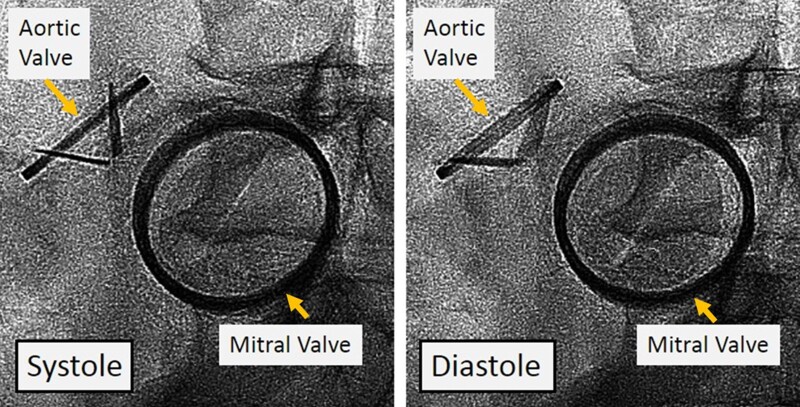
Figure 2.
Case 1. Cine-fluoroscopy of mechanical aortic and mitral valves. Severely reduced leaflet motion of both aortic and mitral valves in systole and diastole.
A multi-disciplinary discussion with critical care cardiology, cardiac surgery, and neurology was conducted with consideration of her NYHA Class III obstructive symptoms and her history of ischaemic stroke with haemorrhagic conversion 3 months prior. Given her severe haemodynamic compromise and that an ischaemic stroke more than 3 months prior is only a ‘relative’ exclusion criterion for full-dose thrombolysis, her intra-cranial bleeding risk was thought to be acceptable despite the prior haemorrhagic conversion, especially given the intended low-dose and slow infusion fibrinolytics. Computed tomography of the brain to rule out ischaemic events was not performed due to a lack of physical exam findings to indicate a stroke. After shared decision-making, the patient opted for a trial of high-risk thrombolysis rather than operative valve intervention. She was given serial low-dose ultra-slow infusions of tissue plasminogen activator (tPA), 25 mg over 24 h, with interval re-initiation of a therapeutic unfractionated heparin drip with goal anti-Xa level of 0.3–0.7 IU/mL for 6 h or an activated prothrombin time (aPTT) of 1.5–2.5 times the control value in seconds while her valve function was assessed. After three serial tPA administrations, her valve gradients relatively improved on TTE, with the mean gradient across both the mitral (11 mmHg) and aortic (27 mmHg) mechanical valves significantly decreased from initial presentation, although persistently abnormal. A subsequent cine-fluoroscopy showed improvement in leaflet mobility of both the aortic and mitral mechanical valves suggesting resolution of the dual PVT. Additionally, she had significant clinical improvement in her heart failure symptoms with return to baseline NYHA Class II. She was then transitioned to warfarin with a goal INR of 2.5–3.5. At the time of discharge, her INR was noted to be therapeutic at 2.7, and she was discharged in stable condition with close ongoing valvular follow-up. The patient re-presented 5 months later in cardiogenic shock due to repeat aortic thrombus from sub-therapeutic INR secondary to medication non-compliance. Trans-oesophageal echocardiography revealed aortic velocity of 429 cm/s with an 11 × 5 mm thrombosis indicating severe aortic stenosis and left ventricular ejection fraction of 55–60%. No mitral valve thrombosis was seen. The patient received veno-arterial extracorporeal membrane oxygenation for haemodynamic support and unfortunately passed away from this episode after surgical valve replacement.
Patient 2
A 43-year-old woman presented with dyspnoea at rest. She had a medical history of bacterial endocarditis followed by a mitral valve repair 16 years prior to presentation, followed by a redo bioprosthetic mitral valve replacement the following year, and eventually a mechanical mitral (25 mm St. Jude, St. Jude, Saint Paul, Minnesota, USA) and mechanical aortic valve (21 mm St. Jude, St. Jude, Saint Paul, Minnesota, USA) replacement 1 year prior to presentation. Notably, her warfarin had been temporarily stopped for a recent dental procedure without bridging anticoagulation but had since been restarted. A TTE noted a left ventricular ejection fraction of 55%, newly reduced right ventricular function, and an increased mean gradient across the mitral valve (23 mmHg), suggestive of severe prosthetic stenosis. The prosthetic aortic valve also appeared to have moderate valvular regurgitation which was not adequately measured on imaging due to the high-velocity mitral inflow into the left ventricular outflow tract limiting the assessment of aortic regurgitation severity (Figure 3). Pertinent labs included elevated liver enzymes, creatinine, and therapeutically elevated INR (3.5). Her HASBLED score was 3 indicating a 5.8% risk of major bleeding with a CHA2DS2VASc score of 2. She underwent a right heart catheterization that revealed a right atrial pressure 10 mmHg, pulmonary artery pressure 64/36 (mean 45) mmHg, pulmonary capillary wedge pressure 33 mmHg, and cardiac index 1.5 L/min/m2. Intravenous unfractionated heparin was avoided due to the elevated INR. Cine-fluoroscopy was performed to assess the valvular function better, demonstrating that both mechanical valves had severely reduced leaflet motion consistent with dual aortic, with enlarged opening angle of 65 degrees, and mitral, with a unmeasurable opening angle, PVT (Figure 4). Intravenous norepinephrine was started for hypotension due to Society for Cardiovascular Angiography and Interventions Stage C cardiogenic shock.
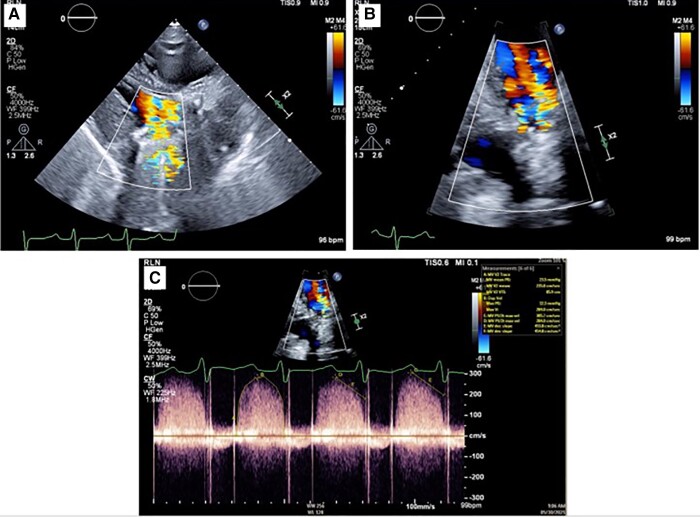
Figure 3.
Case 2. Trans-thoracic echocardiogram of mechanical mitral and aortic valves. (A) Parasternal long-axis view with small left ventricular cavity and colour Doppler mosaic pattern indicating increased velocities of mitral inflow. (B) Apical four-chamber zoomed view of mitral valve with colour Doppler showing mosaic pattern of mitral inflow. (C) Apical four-chamber continuous wave Doppler of mitral valve inflow demonstrating an elevated gradient across the mitral valve, mitral valve mean pressure gradient 23 mmHg, heart rate 89 b.p.m.
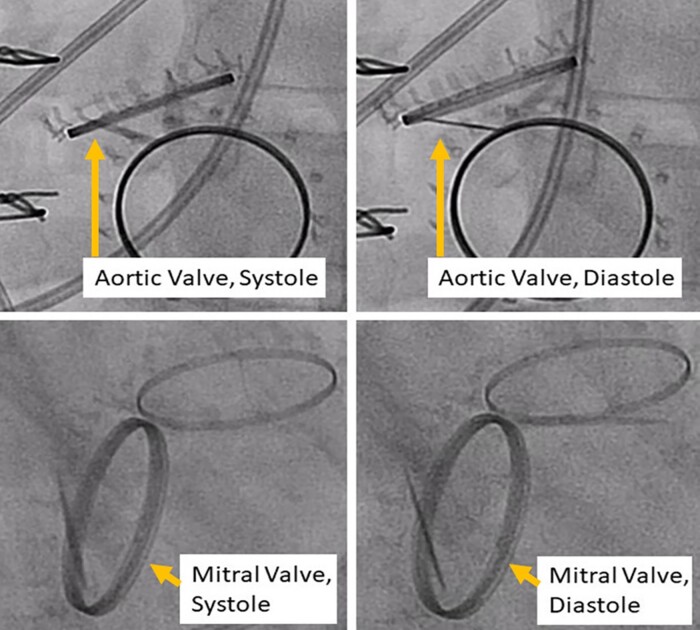
Figure 4.
Case 2. Cine-fluoroscopy of mechanical aortic and mitral valves. Both mechanical valves had severely reduced leaflet motion in both systole and diastole consistent with apparent aortic and mitral prosthetic valve thrombosis.
After multi-disciplinary heart team discussion and shared patient decision-making, she was deemed prohibitive risk for a 4th sternotomy, and, despite the coagulopathy and cardiogenic shock, she was planned for a trial of high-risk thrombolysis. She was initially treated with a slow infusion of tPA 25 mg over 6 h considering her competing risks from unstable haemodynamics and coagulopathy. There was prompt haemodynamic improvement after the initial tPA administration, and norepinephrine was discontinued. Cine-fluoroscopy showed persistently fixed mitral and aortic valve leaflets on Day 2 after tPA administration. Balloon valvuloplasty was considered but not pursued given her overall haemodynamic improvement. Notably, her INR uptrended to 6.9 on hospital Day 1, and she received intravenous Vitamin K 5 mg with normalization of her INR by hospital Day 3. She received two more tPA infusions at an ultra-slow rate, 25 mg over 24 h each, the second on hospital Day 2 with the INR noted to be downtrending to 5.2, and the third on hospital Day 3 with interval intravenous unfractionated heparin given the normalized INR. Serial assessment of valvular function by TTE demonstrated improved mean mitral valve gradient (18 mmHg) and elevated mean aortic valve gradient (66 mmHg) due to improved mitral valve inflow velocity. With echocardiographic improvement and resolution of her obstructive symptoms defined as a return to baseline NYHA Class II, the patient was then transitioned to intravenous unfractionated heparin as a bridge to therapeutic warfarin with a goal aPTT of 1.5–2.5 times the control value in seconds. On Day 5, repeat cine-fluoroscopy showed freely moving aortic valve leaflets and a persistently closed singular mitral valve leaflet. The patient was discharged with a plan for follow-up and possible balloon mitral valvuloplasty if no improvement was seen. Repeat cine-fluoroscopy 2 weeks after discharge showed continued immobility of the mitral valve leaflet; therefore, valvuloplasty was planned. Another 2 weeks later, repeat cine-fluoroscopy prior to planned valvuloplasty showed freely moving mitral valve leaflets reflecting continued valvular improvement while on therapeutic anticoagulation. The patient was then continued on warfarin with goal INR 2.5–3.5 without further intervention, and no subsequent echocardiography was performed.
Discussion
We present two cases of obstructive dual PVT managed with medical therapy over surgical intervention due to complex risk factors. We highlight the use of medical therapy in a minimally examined high-risk population with the need for interdisciplinary and shared decision-making among numerous specialties to tailor treatments based on each patient’s co-morbidities as well as methods to monitor for PVT resolution.
Prosthetic valve thrombosis is a severe adverse event associated with prosthetic valves and is often associated with the temporary cessation of anticoagulation. There is no standard treatment regimen for single-valve PVT, and the success of surgical intervention is variable.9–11 Furthermore, each treatment modality carries unique risks that must be considered in clinical decision-making. Obstructive dual PVT is rare, though likely underreported in the literature. The highest incidence of dual PVT was noted in the PROMETEE trial with nine patients accounting for 7.5% of the studied population.7 Adverse effects are likely higher than single-valve thrombosis due to complexity of the thrombosis, but no studies have analysed this population. Therefore, guidance is based on expert opinion and primarily extrapolated from experience with single-valve PVT.4,12–14 It is essential to confirm the diagnosis of PVT, which can be done by echocardiogram, cine-fluoroscopy, or computed tomography, depending on the clinical history, imaging modality availability and concern for alternative diagnoses, including pannus and endocarditis. Patients with a sub-therapeutic INR should be placed on intravenous unfractionated heparin for therapeutic anticoagulation while treatment options are discussed.3,12
There is limited data to guide thrombolysis in PVT. In the TROIA trial, low-dose, slow infusion of alteplase 25 mg delivered over 6 h showed similar success rates over fast infusion of tPA with less adverse events in a non-randomized comparison of chronologically different treatment groups.15 The subsequent single-arm PROMETEE trial demonstrated 90% treatment success with low-dose, ultra-slow infusion of alteplase 25 mg delivered over 25 h followed by 6 h of intravenous unfractionated heparin with goal aPTT level of 1.5–2.5 times the control value in seconds.7 The non-randomized HATTUSHA study similarly demonstrated higher success rate and lower complication rate with low-dose and slow or ultra-slow infusion of tPA compared to surgery for single-valve thrombosis.8 Notably, the HATTUSHA protocol excluded patients in cardiogenic shock from obstructive PVT as they were not considered candidates for thrombolysis. No trial indicated the limit of repeated tPA dosing or guidelines to define thrombolysis failure. Treatment success was based on improvement of leaflet mobility on imaging including TTE, cine-fluoroscopy, and improvement of NYHA class symptoms.8
Baseline INR before thrombolytic therapy administration varied between the TROIA, PROMETEE, and HATTUSHA studies, ranging from 2 to 3, less than 2.5, and 1.3–2.5, respectively. Anecdotally, an elevated INR is frequently considered a contraindication to thrombolysis. However, the INR limit before thrombolytics can be safely administered has not been defined. Two retrospective series have examined thrombolysis safety in patients with elevated INR between 2 and 5 with a low population of dual valve thrombosis and showed low rates of bleeding complications.16,17 The American Heart Association (AHA) guidelines for treatment of mechanical PVT recommend urgent low-dose, slow-infusion thrombolysis or surgery for left-sided mechanical valves with symptoms of obstruction. The AHA guidelines further state that thrombolysis is ‘an acceptable alternative for obstructive PVT patients at high or prohibitive surgical risk or in patients who have a small thrombus burden, mild HF symptoms (NYHA class I or II), and low bleeding risk’, but do not comment on modifications for high bleeding risk patients or dual PVT.13 The European Society of Cardiology (ESC) guideline recommends surgical intervention or fibrinolytics when surgery is too high risk. The dose of fibrinolytics and the definition of high bleeding risk have not been standardized.14 Per 2020 American College of Cardiology (ACC)/AHA guidelines after resolution of a PVT, anticoagulation decision depends on the mechanism for the PVT. If anticoagulation was inadequate prior to the PVT event, then strict achievement of the standard INR range is appropriate which is the case in both of our patients. Otherwise, an increase in the regimen or target goal should be performed with an addition of an antiplatelet agent as the initial step then gradual increases of the INR target goal.13
To our knowledge, there have been rare reports of dual PVT treatment with four cases reported in patients with an elevated INR above 2.5.8,17 We report two patients with severe mitral and aortic PVT, who presented with NYHA Class III and IV heart failure symptoms, including one with cardiogenic shock. Both patients had elevated bleeding risk, one from coagulopathy established by an elevated INR > 3.0 and the other with prior ischaemic strokes with haemorrhagic conversion; both were successfully treated with low-dose thrombolytics, with slow or ultra-slow infusions. The first patient declined surgical valve intervention and then received a low-dose, ultra-slow tPA infusion. Utilization of tPA was a complex clinical decision only reached after multi-disciplinary discussion and shared decision-making with the patient. Notably the first patient had an ischaemic stroke with haemorrhagic conversion and not a true primary haemorrhagic stroke, and our institutional stroke service felt the intra-cranial bleeding risk from low-dose thrombolysis was not prohibitive with this history. The stroke guidelines in fact state that full-dose tPA can be administered for a recurrent ischaemic stroke, except when the ischaemic stroke occurred within the past 3 months.18 The second patient had an elevated INR (INR level > 3.0) and prohibitive surgical risk, with slow infusion initially chosen due to haemodynamic instability and subsequent ultra-slow infusion once more stable. Utilizing the HATTUSHA trial, we implemented a protocol for serial doses of tPA up to six times until resolution of obstruction was maintained, defined as complete resolution of obstruction or partial resolution with a small residual thrombus <10 mm as well as clinical improvement of NYHA class symptoms back to baseline I and II which were based on the HATTUSHA study resolution endpoints as well as cine-fluoroscopy findings based on the European Association of Cardiovascular Imaging guidelines.8,19 Subsequently anticoagulation treatment could include subcutaneous low-molecular-weight heparin, intermittent subcutaneous unfractionated heparin to achieve an aPTT of 1.5–2.5 times control, or therapeutic warfarin therapy with a target INR of 2.5–3.5 for 3 months. In our limited experience, we have found that low-dose and slow or ultra-slow tPA infusion are safe and effective strategies for high bleeding risk patients with PVT, including dual valve PVT.
Conclusion
Dual mechanical PVT is a rare but serious complication of mechanical valve replacement, and its management should include multi-disciplinary discussion individualized to each patient’s unique risk factors. Patients with obstructive symptoms and high surgical risk or who opt for medical therapy should undergo thrombolysis. Low-dose and slow or ultra-slow tPA infusion should be considered for those with high bleeding risk and can be safe even with elevated INR. Thrombosis may continue to resolve over weeks after thrombolysis, and percutaneous intervention may be considered for refractory valve dysfunction.
Supplementary Material
Acknowledgements
C.N.M. and B.B.K. have full access of all data in the study and take full responsibility in the integrity and accuracy of the data. C.N.M., M.Y., A.P., T.R., and B.B.K. have contributed substantially to the writing, data analysis, interpretation, and accuracy of the manuscript.
Consent: Written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patients in line with COPE guidance.
Funding: None declared.
Contributor Information
Colin McGuire, Department of Medicine, Georgetown University Hospital, Washington, DC, USA.
Michael Yang, Division of Cardiology, Medstar Washington Hospital Center, 110 Irving St., NW, Washington, DC, USA.
Alexander Papolos, Division of Cardiology, Medstar Washington Hospital Center, 110 Irving St., NW, Washington, DC, USA; Department of Critical Care Medicine, Medstar Washington Hospital Center, 110 Irving St., NW, Washington, DC, USA.
Toby Rogers, Section of Interventional Cardiology, MedStar Washington Hospital Center, Washington, DC, USA; Cardiovascular Intervention Program, Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, USA.
Benjamin Kenigsberg, Division of Cardiology, Medstar Washington Hospital Center, 110 Irving St., NW, Washington, DC, USA; Department of Critical Care Medicine, Medstar Washington Hospital Center, 110 Irving St., NW, Washington, DC, USA.
Lead author biography
 Colin N. McGuire is an internal medicine resident at Medstar Georgetown University Hospital, Washington DC. Colin currently works within the cardiovascular institute at Medstar Washington Hospital Center.
Colin N. McGuire is an internal medicine resident at Medstar Georgetown University Hospital, Washington DC. Colin currently works within the cardiovascular institute at Medstar Washington Hospital Center.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports.
Data availability
The data underlying this article are available in the article and in its online Supplementary material.
Prior abstract publication and presentation
McGuire, C., Kenigsberg, B., et. al (2022) ‘Management of Dual Mechanical Valve Thrombosis with Critical Illness and High Bleeding Risk.’ Journal of American Cardiology, 79 (9) (9_Supplement) 3236.
Presented at American College of Cardiology Annual Scientific Session. United States, Washington, D.C. 2022.
References
- 1. Hermans H, Vanassche T, Herijgers P, Meuris B, Herregods M-C, Van de Werf F, et al. Antithrombotic therapy in patients with heart valve prostheses. Cardiol Rev 2013;21:27–36. [DOI] [PubMed] [Google Scholar]
- 2. Roudaut R, Serri K, Lafitte S. Thrombosis of prosthetic heart valves: diagnosis and therapeutic considerations. Heart 2007;93:137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sharif Khan H, Ijaz Z, Ali M, Saif M, Ishaq U, Kamal A, et al. Clinical outcomes of mechanical prosthetic valve thrombosis. Cureus 2020;12:e8760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Biteker M, Altun I, Basaran O, Dogan V, Yildirim B, Ergun G. Treatment of prosthetic valve thrombosis: current evidence and future directions. J Clin Med Res 2015;7:932–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mahindru S, Pande S, Malhotra P, Thukral A, Kotwal AS, Gupta RP, et al. Mechanical prosthetic valve thrombosis in current era: 5-year follow-up. Indian J Thorac Cardiovasc Surg 2021;37:140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dürrleman N, Pellerin M, Bouchard D, Hébert Y, Cartier R, Perrault LP, et al. Prosthetic valve thrombosis: twenty-year experience at the Montreal heart institute. J Thorac Cardiovasc Surg 2004;127:1388–1392. [DOI] [PubMed] [Google Scholar]
- 7. Özkan M, Gündüz S, Gürsoy OM, Karakoyun S, Astarcıoğlu MA, Kalçık M, et al. Ultraslow thrombolytic therapy: a novel strategy in the management of PROsthetic MEchanical valve thrombosis and the prEdictors of outcomE: the ultra-slow PROMETEE trial. Am Heart J 2015;170:409–418. [DOI] [PubMed] [Google Scholar]
- 8. Mehmet Özkan MD, Sabahattin Gündüz MD, Ahmet Güner MD, Macit Kalçık MD, Mustafa Ozan Gürsoy MD, Begüm Uygur MD, et al. Thrombolysis or surgery in patients with obstructive mechanical valve thrombosis: the multicenter HATTUSHA study. J Am Coll Cardiol 2022;79:977–989. [DOI] [PubMed] [Google Scholar]
- 9. Roudaut R, Lafitte S, Roudaut M-F, Reant P, Pillois X, Durrieu-Jaïs C, et al. Management of prosthetic heart valve obstruction: fibrinolysis versus surgery. Early results and long-term follow-up in a single-centre study of 263 cases. Arch Cardiovasc Dis 2009;102:269–277. [DOI] [PubMed] [Google Scholar]
- 10. Nagy A, Dénes M, Lengyel M. Predictors of the outcome of thrombolytic therapy in prosthetic mitral valve thrombosis: a study of 62 events. J Heart Valve Dis 2009;18:268–275. [PubMed] [Google Scholar]
- 11. Huang G, Schaff HV, Sundt TM, Rahimtoola SH. Treatment of obstructive thrombosed prosthetic heart valve. J Am Coll Cardiol 2013;62:1731–1736. [DOI] [PubMed] [Google Scholar]
- 12. Gürsoy MO, Kalçık M, Yesin M, Karakoyun S, Bayam E, Gündüz S, et al. A global perspective on mechanical prosthetic heart valve thrombosis: diagnostic and therapeutic challenges. Anatol J Cardiol 2016;16:980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation 2021;143:e72–e227. [DOI] [PubMed] [Google Scholar]
- 14. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2022;43:561–632. [DOI] [PubMed] [Google Scholar]
- 15. Özkan M, Gündüz S, Biteker M, Astarcioglu MA, Çevik C, Kaynak E, et al. Comparison of different TEE-guided thrombolytic regimens for prosthetic valve thrombosis: the TROIA trial. JACC Cardiovasc Imaging 2013;6:206–216. [DOI] [PubMed] [Google Scholar]
- 16. Nishanth KR, Shankar M, Srinivasa KH, Manjunath CN, Ravindranath KS. Fibrinolysis in left-sided mechanical prosthetic valve thrombosis with high INR. Eur Heart J Acute Cardiovasc Care 2020;9:S58–S62. [DOI] [PubMed] [Google Scholar]
- 17. Farzaneh K, Mortazavi SH, Oraii A, Abbasi K, Salehi Omran A, Ahmadi Tafti SH, et al. Safety of thrombolytic therapy in patients with prosthetic heart valve thrombosis who have high international normalized ratio levels. J Card Surg 2020;35:2522–2528. [DOI] [PubMed] [Google Scholar]
- 18. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019;50:e344–e418. [DOI] [PubMed] [Google Scholar]
- 19. Lancellotti P, Pibarot P, Chambers J, Edvardsen T, Delgado V, Dulgheru R, et al. Recommendations for the imaging assessment of prosthetic heart valves: a report from the European Association of Cardiovascular Imaging endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016;17:589–590. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online Supplementary material.