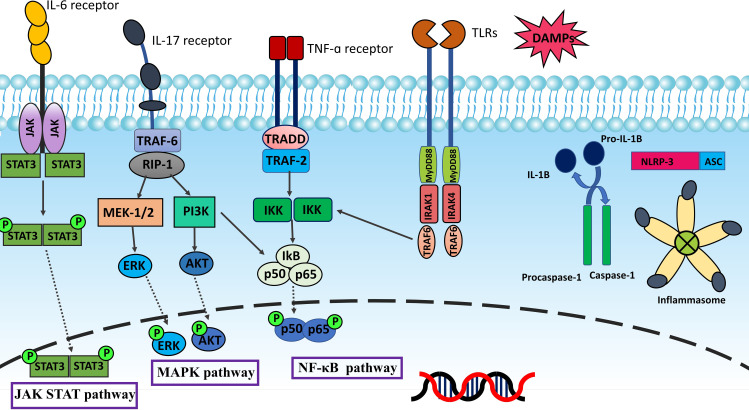
Figure 1.
Inflammation is mainly regulated by NF-κB, MAPK, and JAK-STAT pathways. TLRs and inflammatory cytokines such as TNF-α and IL-17 activate NF-κB pathway via IκB kinase (IKK). The proteasome degradation of phosphorylated IκB releases NF-κB for nuclear translocation and gene transcription activation. The MAPK pathway provides intracellular signaling triggered by external stimuli by phosphorylating and activating MAPK kinases like MEK1/2. MAPK kinases phosphorylate various proteins, including transcription factors like ERK1/2, and phosphorylated ERK translocate to the nucleus resulting in the transcription of inflammatory mediators. Upon IL-6 binding, the IL-6 receptor transduces the signal to activate JAKs, then JAKs phosphorylate the receptor. The phosphates are subsequently bound by two STAT proteins, which JAKs then phosphorylate to create a dimer. The dimer penetrates the nucleus, binds to DNA, and causes target genes to be transcribed. When PAMPs and DAMPs trigger the innate immune system and inflammatory signaling, NLRP3 forms oligomers and activates caspase-1. This starts the processing and release of IL-1β, which is a pro-inflammatory cytokine. Inflammasomes are sensors of the innate immune system that regulate the activation of caspase-1 and generate inflammation. As DAMPs stimulate the innate immune system, NLRP3 starts oligomerization and activation of caspase-1, causing the release of the pro-inflammatory cytokine IL-1β.