Figure 4.
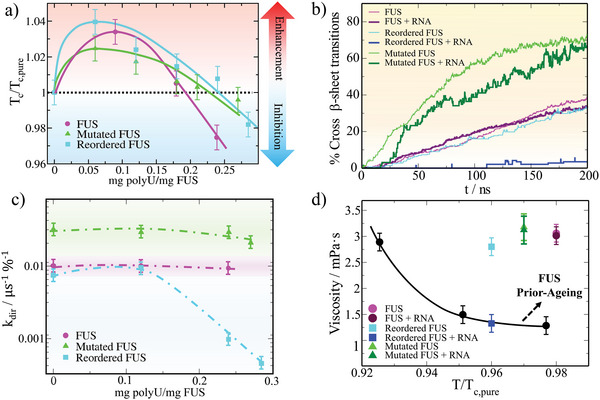
RNA at moderate concentration inhibits the formation of inter‐protein β‐sheet clusters in sequence domain‐reordered FUS condensates. a) Critical temperature (filled symbols) of FUS (magenta), mutated FUS (green), and reordered FUS (cyan) condensates as a function of the polyU/protein mass ratio. Symbols above the horizontal dotted line indicate RNA‐driven LLPS enhancement while symbols below such threshold denote inhibition of condensate phase‐separation. Continuous curves are included as a guide for the eye. Please note that temperatures have been renormalized by the critical temperature of each sequence in absence of RNA (T c, pure ). b) Time‐evolution of the percentage of LARKS forming crossed‐β‐sheet motifs in phase‐separated condensates of the three FUS variants both in absence and presence of RNA as indicated in the legend. The polyU/protein mass ratio of each condensate corresponds to 0.24, 0.27, and 0.28 for FUS, mutated FUS and reordered FUS, respectively (i.e., that is the maximum polyU concentration at which condensates are stable above T/T c, pure ≈0.97; temperature at which all simulations were performed). c) Estimated kinetic constants (from a second‐order reaction analysis to the number of inter‐protein β‐sheet transitions over time) for the different polyU/FUS condensates at increasing polyU/protein mass ratios at T ≈0.97 T c, pure . Error bars account for the estimated uncertainty while dotted lines are included as a guide for the eye. d) Viscosity as a function of temperature for pure FUS condensates before the emergence of inter‐protein β‐sheet transitions (black circles), and after a maturation time interval of 0.3 µs for each FUS variant both in absence and presence of RNA as indicated in the legend. Temperature has been renormalized by T c, pure of each FUS variant. The polyU/protein mass ratios of the mixed condensates are the same as those described in Panel (b).
