Figure 5.
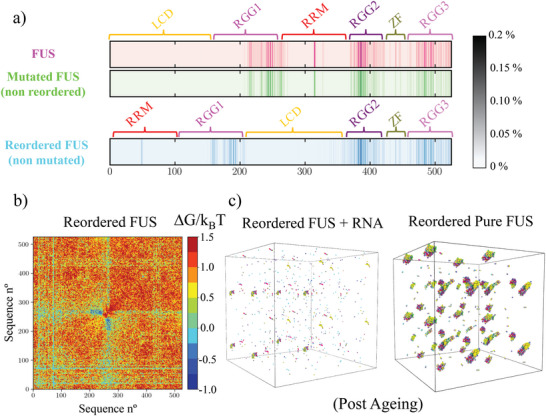
The location of the strong RNA‐binding domains in FUS is compelling to inhibit disorder‐to‐β‐sheet transitions at moderate RNA concentrations. a) Average contact probability per residue (scale bar) between polyU and the different amino acids composing the three FUS variants in non–aged phase‐separated condensates at T/T c, pure ≈0.97 and the maximum polyU/protein mass ratio at which condensates are stable at such temperature (i.e., 0.24 for FUS, 0.27 for mutated FUS, and 0.28 for the reordered FUS variant). b) Free energy inter‐molecular variation computed from the molecular contact probability between aged reordered FUS pure condensates and polyU/reordered FUS ones at a polyU/protein mass ratio of 0.28, T ≈0.97 T c, pure , and after an ageing time interval of 0.3 µs for both systems. c) β‐sheet network connectivity (evaluated through the PPA method) of an aged polyU/reordered FUS condensate (Left) and a pure reordered FUS condensate (Right) upon 0.3 µs of maturation.
