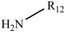Table 1.
Summary of Kaol modification methods
| Categories | Modifier | Molecular structure | Dipole moment [D] | Basal spacing [nm] | Intercalate ratio [%] | Action sites | Synthesis method | Refs. |
|---|---|---|---|---|---|---|---|---|
| Surface modification | TBSCI |
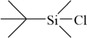
|
— | — | — | Formation of H—O—Si hydrogen bonds through Si with oxygen atoms of Si—OH tetrahedra | Atmospheric reaction | [93] |
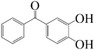
| 3,4‐dihydroxybenzophenone |
|
— | — | — | Formation with Al3+ of the aluminol basal surface | Solution | [100] | |
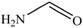
| Intercalation | formamide |
|
3.71 | 1.06 | 92.10 | Si—OH and O—H on the inner surface form hydrogen bonds with NH and C=O, and NH was partially inserted into the Si—OH tetrahedra | Homogenization, solution | [101, 102] |
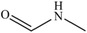
| N–methylformamide (NMF) |
|
3.83 | 1.08 | 94.00 | Formation of NH—O—Si hydrogen bonds through NH with oxygen atoms of Si—OH tetrahedra | Solution | [102, 103] | |
| hydrazine |

|
1.75 | 1.04 | 59.00 | Four hydrogen atoms of hydrazine with Si—OH interlayer | Solution | [104] | |
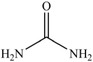
| urea |
|
4.56 | 1.07 | 92.00 | C=O and N—H form hydrogen bonds with the Al—OH interlayer, and the O of the Si—OH interlayer | Solution, aqueous suspension, homogenization | [102, 105] | |
| dimethyl sulfoxide (DMSO) |
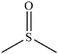
|
3.96 | 1.12 | 94.40 | The C—S—C and CH3 are parallel to the Al interlayer, with one CH3 pointing to the center of the Si—OH tetrahedral | Solution, homogenization method | [106, 107] | |
| Grafting modification | pyridine |
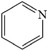
|
2.19 | 1.22 | 90.20 | N form hydrogen bonds with hydroxyl groups on the Al—OH octahedron | — | [108] |
| potassium acetate (Kac) |
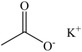
|
— | 1.42 | 73.00 | O on the carboxyl group form a hydrogen bond with the hydroxyl group of Al—OH octahedron | Solution | [109] | |
| methanol (MeOH) |

|
1.70 | 0.85 | 96.00 | O—H with Al—O to form an Al—OH and H2O; an Al—O—C is formed by O—H and O of Al—OH interlayer | Solvothermal | [110] | |
| 3,4‐dihydroxybenzophenone (APTES) |
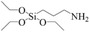
|
— | 1.73–1.89 | 95.00 | Electrostatic interactions between N—H and O of Al—OH interlayer | Solution/stirring, solvothermal | [111, 112, 113] | |
| Exfoliation modification | ammonium salts |
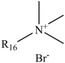
CTAB
|
— | 2.07 | 65.62 | Si—O—Si groups interacted with CTAB+ cations | One‐step delamination | [114] |
|
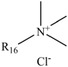
CTAC
|
— | 3.79 | 79.00 | — | Solvothermal | [82] | ||
|
DDA
|
— | 4.16 | 70.00 |
Interlayers in both paraffin‐type structures and distorted conformation Solution |
Solution | [81] |