Figure 2.
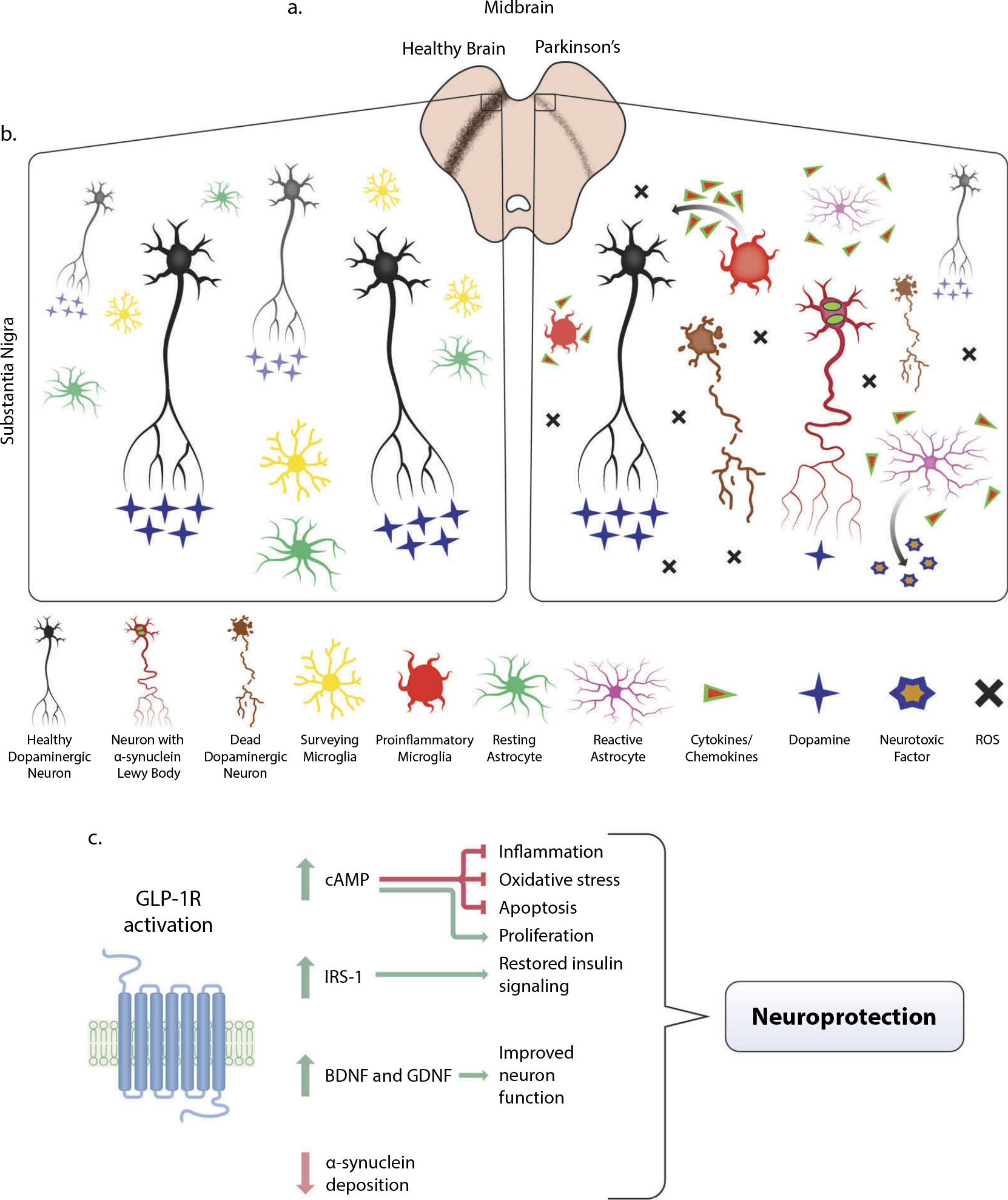
Pathology of Parkinson’s Disease (PD) in the midbrain and mitigation through GLP-1R activation. (a) In the midbrain of PD patients, loss of dopaminergic neurons is visible via decreased immunostaining of tyrosine hydroxylase (TH) (brown), the precursor to dopamine. In animal models of PD, including the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and 6-hydroxydopamine (6-OHDA) lesion methods, similar loss of dopaminergic neurons in the midbrain is observed. (b) A microenvironment comparison of a healthy and PD afflicted midbrain. Dopaminergic neuron dysfunction, reduced dopamine transmission, α-synuclein deposition within neurites, and eventual death may arise from a variety of genetic or environmental factors that cause mitochondrial dysfunction and high amounts of oxidative stress. The accumulation of reactive oxygen species (ROS), neuronal α-synuclein, and dying cells are some of the components that contribute to a proinflammatory environment in the PD midbrain. Highly dynamic surveying microglial cells respond to this milieu by altering their activation state and producing proinflammatory cytokines and chemokines that cascade to further evoke reactive astrocyte activation from their resting quiescent state. These astrocytes secrete a neurotoxic factor that selectively ablates subsets of neurons and oligodendrocytes and further exacerbates the already chronically inflamed region. Though inflammation is an immune response necessary for repair, chronic inflammation is especially detrimental. (c) Upon activation of GLP-1 receptor (GLP-1R), a multitude of downstream pathways are activated that mitigate the effects of PD pathology, most notably the major upstream secondary messenger cyclic adenosine monophosphate (cAMP). Upregulation of cAMP induces downstream effector proteins that ameliorate inflammation, oxidative stress, and apoptosis, which provides neuroprotection and proliferative capabilities for neurite outgrowth (see Athauda and Foltynie, 2016b and Glotfelty et al., 2019 for more detailed signaling pathways). Restoration of insulin signaling through the upregulation of active insulin receptor substrate-1 (IRS-1) and downstream proteins provide additional neuroprotection (see Athauda and Foltynie, 2016a and Hölscher, 2020). This, coupled with increased production of brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF), contributes to the amelioration of deficits associated with PD.
