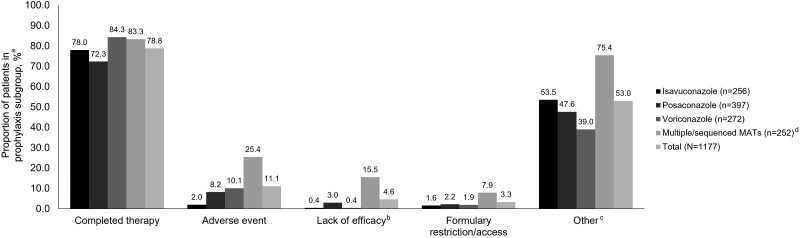
Figure 1.
Reasons for the discontinuation of mold-active triazoles in the prophylaxis subgroup (safety analysis set). Patients could have been counted in multiple categories but were only counted once for each category. aPercentages are derived from the number of patients without missing data in each category; isavuconazole n = 245, posaconazole n = 368, voriconazole n = 267, multiple/sequenced MATs n = 252, and total n = 1132. bLack of efficacy was reported for a patient who received MAT prophylaxis, developed a bIFD, and then switched to a new MAT for treatment. c“Other” reasons for discontinuation of MATs included but were not limited to hospital visits, hospital admission, discharge, lack of intravenous formulation, and inpatient and outpatient switching. dMultiple/sequenced MATs described patients receiving >1 MAT for prophylaxis throughout the study since index/enrollment. Abbreviations: bIFD, breakthrough invasive fungal infection; MAT, mold-active triazole.