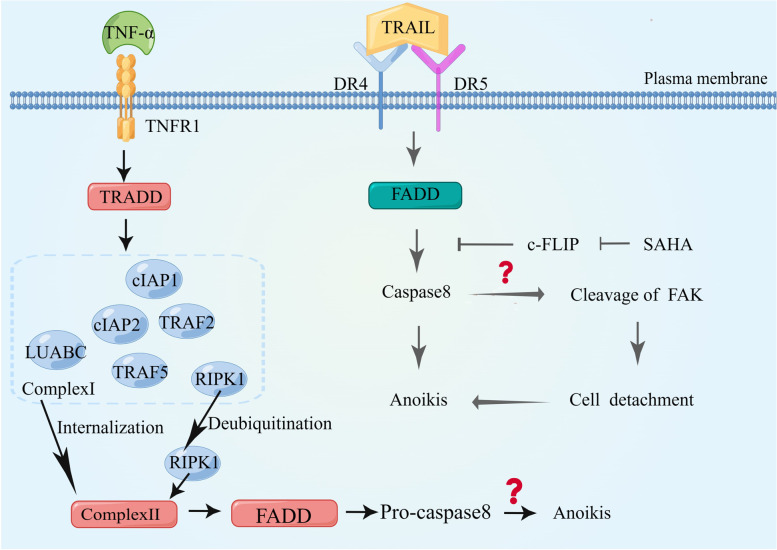
Fig. 6.
Possible mechanisms of anoikis mediated by TNFR1 and DR4/DR5. TNF-α binding to TNFR1 leads to the formation of different signaling complexes that define different cell fates. TNF-α binding to TNFR1 recruits TNF receptor-associated proteins and the death domain (TRADD). TRADD forms signal complex I with TRAF2, TRAF5, RIPK1, LUBAC, cIAP1, and cIAP2. Complex I internalizes and transforms into the death-inducing complex, Complex II, with additional recruitment of FADD and pro-caspase8. Many outcomes of TNFR1 activation depend on post-translational modification of Complex I member RIPK1; deubiquitination of RIPK1 facilitates its release from Complex I and binding to Complex II to induce apoptosis. DR5 and DR4 can participate in anoikis, but the specific mechanism remains to be clarified