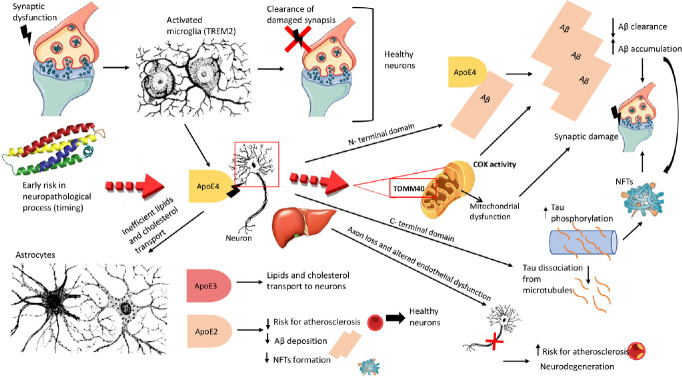
Figure 1.
Apolipoprotein E (ApoE)-mediated development of Alzheimer’s disease-type neuropathology.
Synapse dysfunction induces the activation of microglia, which removes damaged synapses in healthy neurons. Apolipoprotein E (APOE) alleles differentially impact glial responses. Triggering receptor expressed on myeloid cells 2 (TREM2) protein could be an important mediator. The inefficient transport of lipids and cholesterol from astrocytes, and microglia activation (including TREM2 activation) could be conditioned by the APOE ε4 allele. The stress and injury condition stimulate the neurons to release ApoE4, which with its N-terminal domain binds amyloid-beta (Aβ), promoting a reduction in clearance and accumulation of Aβ, leading to the formation of Aβ plaques and accentuating the synaptic damage. The C-terminal domain of ApoE can interact with neurofibrillary tangles (NFTs), which originate from tau hyperphosphorylation and successive dissociation of tau from microtubules provocating axon loss and neurodegeneration. This mechanism seems to be principally consequent to Aβ. ApoE4 isoform released by the central nervous system and peripherally by hepatocytes increases the risk for atherosclerosis, while ApoE2 and ApoE3 (intermedius) decrease this risk because of its protective effect in sporadic Alzheimer’s disease (decreases Aβ deposition and NFT formation). In addition, a dynamic mitochondrial dysfunction could be induced by the ApoE4 interaction with the translocase of the outer mitochondrial membrane 40 (TOMM40) gene on the outer mitochondrial membrane. Compared to noncarriers, APOE ε4 carriers have lower mitochondrial cytochrome oxidase (COX) activity in posterior cingulate cortex neurons, also without soluble or insoluble Aβ or tau pathology. This could be considered a very initial change that come first and prompted β-site amyloid-β precursor protein-cleaving enzyme (BACE1) activity and amyloid genesis. Created using Microsoft PowerPoint 2019.