Bielamowicz K, Fousek K, Byrd TT, Samaha H, Mukherjee M, Aware N, Wu MF, Orange JS, Sumazin P, Man TK, Joseph SK, Hegde M, Ahmed N. Trivalent CAR T cells overcome interpatient antigenic variability in glioblastoma. Neuro Oncol. 2018 Mar 27;20(4):506-518.
doi: 10.1093/neuonc/nox182.
The authors of this article wish to amend the data shown in Fig. 6 that were found to be erroneous. The new figure is shown here along with the corresponding methods and a new description of the pertinent results. The new data do not change the conclusions or impact of the study. The authors apologize for the error.
Fig. 6.
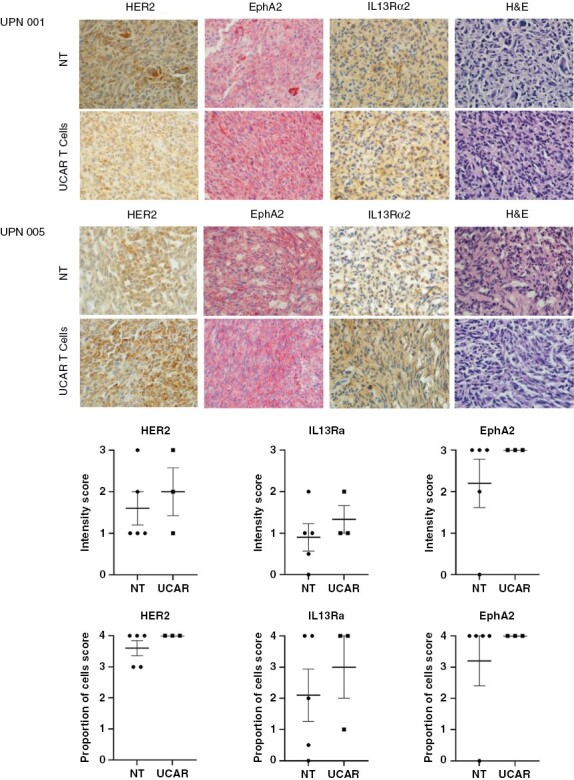
(A) Immunohistochemistry of UPN001 and UPN005 xenografts from frozen mouse brain sections after treatment with T cells when mice reached the humane point for euthanasia. GBM tumor xenografts of mice (N = 3-5 per group) from the Non-transduced (NT) and UCAR T-cell treated groups were characterized post treatment using immunohistochemistry staining for HER2, EphA2, and IL13Rα2, DAPI, at 40x magnification. Representative images shown. (B) Graphic representation of HER2, EphA2, and IL13Rα2 positively stained cells in brain sections of mice quantified collectively from 40x high power fields in each group. Each data point represents a specimen examined for intensity of staining and proportion of cells showing positivity for the examined antigen. Intensity: 1+ (weak); 2+ (moderate); 3+ (strong). Proportion of cells staining: 1+ (1-25%); 2+ (>25-50%); 3+ (>50-75%); 4+ (>75%). Error bars represent the Mean +/- SEM.
Methods:
Murine brains were cryopreserved in OCT compound and stored at -80C. Slides of cryostat cuts were prepared. H&E was done for each sample on a Gemini AS (Thermofisher). HER2 (1:10, Abcam #ab8054) staining was performed by antigen retrieval using EDTA and overnight staining at 4C followed by Vector Laboratories ImmPRESS Mouse kit. IL13-Rα2 (1:100, Proteintech #11059-1) and EphA2 (1:1000, Santa Cruz #sc-398832) was performed on the BONDIII automated system with antigen retrieval respectively (H1) for 20 minutes and (H2) at pH 9 for 20 minutes, and BOND Polymer Refine Red Detection Kit. H&E staining was evaluated for xenograft human tumor tissue with representative images of IHC staining in tumor-containing areas of subsequent IHC slides by a clinical neuropathologist. Images were taken at 40x magnification.
Results:
We characterized the expression of glioma antigens HER2, EphA2, and IL13Rα2 on progressive mice explants of PDXs UPN001 and UPN005 post treatment and at the humane endpoint for euthanasia (Fig. 6). In a control group treated with NT T cells, we found that these progressive tumor xenografts had variable levels of all 3 antigens. Post UCAR T-cell treatment, the recurrent tumors continued to have variable expression of the 3 antigens, with no notable statistical significant between groups.
Authorship
There are also two authors to be added to the byline:
Carrie Mohila, MD, PhD. Associate Professor of Immunology and Pathology, Baylor College of Medicine. American Board of Pathologists certified in Neuropathology and Anatomic and Clinical Pathology
John Hicks, MD, PhD. Professor of Pathology & Immunology, and Pediatrics, Baylor College of Medicine


