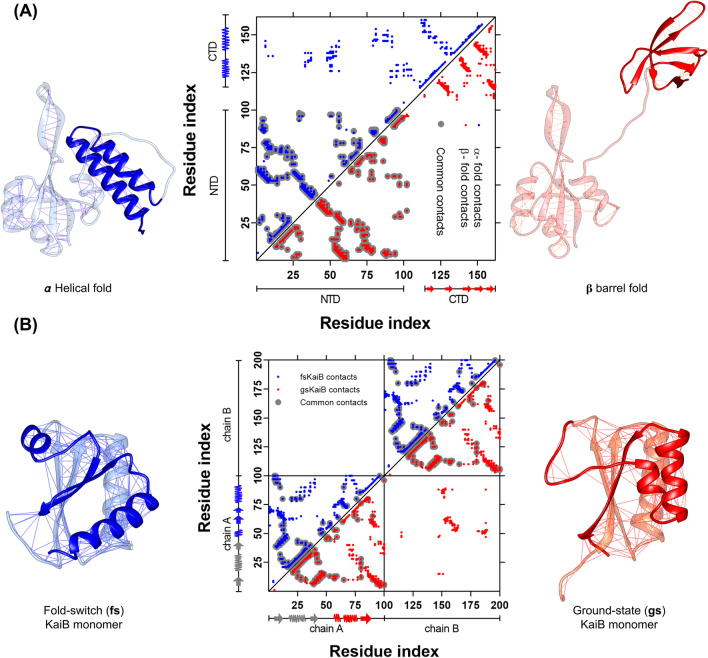
Fig. 1.
Topological rearrangement due to the fold-switch of the metamorphic proteins RfaH and KaiB. A Cartoon representation of full-length RfaH, with the C-terminal domain (CTD) folded as an α-helical hairpin (PDB 5OND, blue) or a β-barrel (PDB 2LCL, red), with the lines on each structure representing the native contacts. The middle panel shows the residue pair contact map for each native state of RfaH CTD, with the upper left triangle corresponding to the native contacts in the α-helical fold (blue) and the lower right triangle to the β-barrel fold. The interdomain contacts can be seen in the upper part of the contact map. B Cartoon representation of the KaiB monomer in the fold-switch (fs) state (PDB 5JYT, blue) and the ground-state (gs) fold (PDB 1VGL, red), with the lines on each structure representing the native contacts, highlighting the C-terminal half of the monomer that experiences the topological rearrangement. The middle panel shows the residue pair native contact map for each native state, with the upper left triangle corresponding to the native contacts in the fs state (blue) and the lower right triangle to the gs fold (red). Common contacts between both folds are shown in gray. Given that the gs fold is only observed in KaiB dimers or tetramers, the lower right square presents the intermolecular interactions between adjacent subunits in the KaiB dimer