Fig. 8. The speculative mechanism for ATP and PPP to induce protein folding.
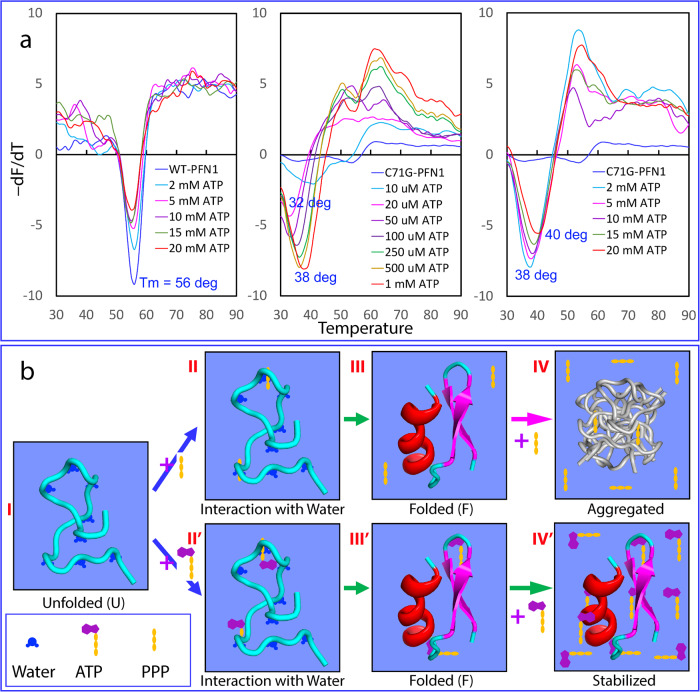
a ATP enhances thermodynamic stability of C71G-hPFN1 but not WT-hPFN1 as measured by differential scanning fluorimetric (DSF). Melting curves of thermal unfolding of WT-hPFN1 and C71G-hPFN1 in the presence of ATP at different concentrations by plotting the first derivative of the fluorescence emission as a function of temperature (−dF/dT). Here Tm is represented as the lowest point of the curve. b A speculative model for protein folding induced by triphosphate (PPP) and ATP. For the unfolded state (U) of a protein, many backbone atoms are hydrogen-bonded with water molecules. Both triphosphate (PPP) and ATP can use their triphosphate chain to effectively attract water molecules out from hydrogen-bonding with the backbone atoms, thus favoring the formation of the folded state (F). However, due to the electrostatic screening effects produced by highly charged triphosphate, a protein exemplified by C71G-hPFN1 and nascent hSOD1 with significant exposure of hydrophobic patches due to the defects in tertiary packing will become severely aggregated. By contrast, ATP can use its aromatic base ring to dynamically interact with the exposed hydrophobic patches, which consequently not only functions to prevent aggregation but also to increase the thermodynamic stability.