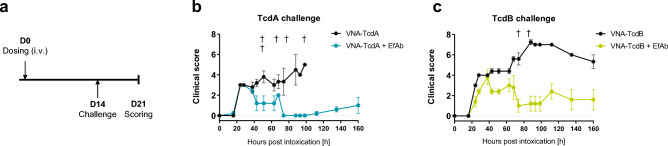
Figure 4.
Co-administration of mRNA-LNP encoded EfAb prolongs the protective activity of VNAs. (a) Illustration of dosing and challenge regimen. Outbred CD-1 mice received a single intravenous injection of mRNA-LNP. After 14 days, animals were challenged with 50 ng of either TcdA or TcdB toxin. Following challenge, animals were scored for clinical symptoms for a period of 160 h (day 21 post injection). (b) Long-term protective efficacy of mRNA-LNP-encoded VNAs and co-administered EfAb in mice exposed to TcdA challenge according to schedule illustrated in (a). Mice which received 2.5 µg of mRNA-LNP encoding VNA-TcdA either alone (grey) or together with 10 µg of mRNA-LNP encoding EfAb (blue) were scored for symptoms of toxemia (Supplement Table 3) and euthanized when exceeding the humane endpoint. Error bars represent the SEM of five mice per group. †Individual animals which succumbed to toxin challenge (group indicated by color). (c) Long-term protective efficacy of mRNA-LNP-encoded VNAs and co-administered EfAb in mice exposed to TcdB challenge according to schedule illustrated in a). Mice which received 2.5 µg of mRNA-LNP encoding VNA-TcdB either alone (grey) or together with 10 µg of mRNA-LNP encoding EfAb (green) were scored for symptoms of toxemia (Supplement Table 3) and euthanized when exceeding the humane endpoint. Error bars represent the SEM of five mice per group. †Individual animals which succumbed to toxin challenge (group indicated by color).