Summary
Background
Glypican-3 (GPC3) is a well-characterized hepatocellular carcinoma (HCC)-associated antigen and a promising target for HCC treatment. CT017 CAR T cells were engineered to co-express CAR-GPC3 and runt-related transcription factor 3 (RUNX3), which triggers CD8+ T-cell infiltration into the cancer microenvironment.
Methods
This single-center, single-arm, open-label, phase I clinical study enrolled heavily pretreated patients with GPC3-positive HCC between August 2019 and December 2020 (NCT03980288). Patients were treated with CT017 CAR T cells at a dose of 250 × 106 cells. The primary objective was to assess the safety and tolerability of this first-in-human product.
Findings
Six patients received 7 infusions (one patient received 2 infusions) at the 250 × 106 cells dose. Three patients received CT017 monotherapy, and three patients received CT017-tyrosine kinase inhibitor (TKI) combination therapy at the first infusion. One patient received CT017-TKI combination therapy at the second infusion after CT017 monotherapy. All patients experienced cytokine release syndrome (CRS), with 50% (3/6) at Grade 2, 50% (3/6) at Grade 3, and all events resolved after treatment. No immune effector cell-associated neurotoxicity syndrome was observed. Dose escalation was not performed due to the investigator’s decision regarding safety. Of six evaluable patients, one achieved partial response and two had stable disease for a 16.7% objective response rate, 50% disease control rate, 3.5-month median progression-free survival, 3.2-month median duration of disease control, and 7.9-month median overall survival (OS) with 7.87-month median follow-up. The longest OS was 18.2 months after CT017 infusion.
Interpretation
Current preliminary phase I data showed a manageable safety profile and promising antitumor activities of CT017 for patients with advanced HCC. These results need to be confirmed in a robust clinical trial.
Funding
This study was funded by CARsgen Therapeutics Co., Ltd.
Keywords: CAR T-cell therapy, Hepatocellular carcinoma, Safety of CAR T-cell therapy in HCC, GPC3-targeted CAR T cells, Immunotherapy for advanced HCC
Research in context.
Evidence before this study
Though systemic therapies for advanced hepatocellular carcinoma (HCC) improve prognosis, long-term clinical benefits are still limited. CAR T cells showed significant efficacy in treating hematologic malignancies but its application in solid tumors is still challenging.
Earliest preclinical study reported the anti-tumor activity of Glypican-3 (GPC3)-targeted CAR T cells by efficiently eliminating GPC3-positive hepatocellular carcinoma and might become a new treatment method for GPC3-positive hepatocellular carcinoma. Preclinical studies of this product showed that Runt-related transcription factor 3 (RUNX3) co-expression improved the persistence, increased T cell infiltration in tumor tissues, and enhanced antitumor activity of CAR T cells in vivo.
Added value of this study
Our study provides the first clinical evidence of newly engineered CAR T cells named CT017 that can co-express GPC3 CAR and RUNX3 for treating advanced HCC patients. The study results showed a manageable safety profile of CT017 and potential therapeutic benefits of CT017 plus TKIs combination therapy in patients with advanced HCC.
Implications of all the available evidence
Advanced-stage HCC is unsuitable for local therapy and available systemic therapeutics provide modest clinical benefit. Current study data provided preliminary evidence that CT017 CAR-GPC3 T cell therapy as monotherapy or combination therapy could be a safe and alternative therapeutic option for advanced HCC patients.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers with substantial morbidity and mortality.1 Many patients are diagnosed with advanced-stage, unresectable disease. Several multi-target tyrosine kinases inhibitors (TKIs, i.e., sorafenib, lenvatinib, regorafenib) have shown favorable survival benefits in HCC since 2008.2, 3, 4 The emerging immunotherapy or immuno-oncology combinations have revolutionized the treatment scenario of solid tumors.5 Immune checkpoint inhibitors (i.e., anti-PD-1/PD-L1 antibodies, anti-CTLA-4 antibodies) as dual-blockade therapy or in combination with TKIs and/or anti-angiogenesis agents have also improved the prognosis for HCC patients in recent years.6, 7, 8, 9 And more benefits are expected for HCC patients with continued explorations on the predictive biomarkers for precision medicine and new combinations of immune checkpoint inhibitors.10, 11, 12 However, the long-term survival of most patients remains poor, and therapeutic options with novel mechanisms are urgently needed.
Chimeric antigen receptor (CAR) T cell therapy is a novel immunotherapy that enables autologous T cells to express synthetic receptors to specifically recognize the surface tumor-associated antigens for exerting subsequent antitumor effects, and eliminating the resistance, metastases, and recurrence of cancer.13 CAR T-cell therapy has achieved remarkable efficacy in the treatment of hematologic malignancies.14,15 However, the application of CAR T cells to solid tumors has been a great challenge. Although the antitumor efficacy was observed in some early phase clinical trials, it was much less effective.16,17 Challenges include finding a safe and effective target, improving CAR T-cell trafficking to the site of disease and increasing the infiltration, and maintaining a durable proliferation of CAR T cells in the tumor microenvironment.18,19
There are several preclinical and clinical studies of CAR T-cell therapies for HCC, demonstrating promising therapeutic potential.20, 21, 22 Glypican-3 (GPC3) is a cell-surface glycophosphatidylinositol-anchored protein. It is extensively and highly expressed on HCC cells and shows minimal expression in normal adult tissues.23 Moreover, elevated GPC3 in HCC is associated with poor prognosis.24 Therefore, GPC3 is one of the well-characterized HCC-associated antigens and is a promising target for the treatment of HCC. Previously, a phase I trial of Y035 CARGPC3 T-cell therapy for advanced HCC showed an expected safety profile and promising antitumor activities with two partial response (PR) and one stable disease (SD) observed in 13 patients.25 Synergistic effects with sorafenib has been studied and demonstrated the potential benefit of combining sorafenib with GPC3-targeted CAR T cells against HCC in preclinical models.22 A patient with recurrent HCC achieved long-term complete response after receiving a combination of sorafenib with four doses of Y035 CAR-GPC3 T cells, which suggests a promising option for patients with advanced HCC.26
To further enhance the efficacy, new technologies to improve GPC3-specific CAR T-cell therapy are being continuously explored. Runt-related transcription factor 3 (RUNX3) is a key regulator of tissue-resident memory (TRM) cell differentiation and homeostasis.27 It functions to trigger CD8+ T-cell infiltration in the cancer microenvironment by regulating a series of immune-related chemokines.28 Whether CAR T cells co-expressing RUNX3 could better infiltrate into tumor tissue and have enhanced antitumor activity remains unknown. Therefore, we engineered new CAR T cells named CT017, which co-express CAR-GPC3 and RUNX3, termed a fourth-generation CAR T-cell therapy. The RUNX3 expression of Run-CAR-T (i.e., CAR T cells co-expressing RUNX3) was measured by Western blot analysis after activation in preclinical studies, and the RUNX3 expression had been well confirmed. Preclinical studies also showed that RUNX3 co-expression improved the persistence, tumor infiltration, and antitumor activity of CAR T cells in vivo.29 In this study, we conducted a prospective phase I trial to explore the safety and potential efficacy of CT017 CAR T cells as monotherapy and combination therapy in patients with heavily treated advanced GPC3 positive HCC.
Methods
Study design and participants
This single-center, open-label, single-arm, phase I trial was held in the First Affiliated Hospital of Zhejiang University in China (NCT03980288). Patients were eligible if they were aged 18–75 years, had a histological diagnosis of HCC with positive GPC3 expression (IHC staining in any percentage of tumor cells with intensity ≥1+), and measurable advanced disease as defined by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Patients were refractory to standard systemic treatment. GPC3 immunohistochemistry staining was evaluated by the central lab as previously described.25 Other key eligibility criteria included a Child-Pugh score ≤7, an Eastern Cooperative Oncology Group (ECOG) performance state score of 0 or 1, HBV-DNA <2000 IU/mL, and adequate bone marrow, liver, and kidney function.
Enrolled patients underwent leukapheresis to obtain peripheral blood mononuclear cells. The manufacturing of CT017 CAR T cells was completed around 2 weeks afterward. CT017 CAR T cells consisted of a humanized anti-GPC3 single-chain variable fragment (scFv) (Supplementary Table S1), CD8α hinge domain, CD8α transmembrane domain, 4-1BB intracellular domain, and CD3ζ intracellular signaling. In addition, by using a self-cleaving F2A peptide, RUNX3 was co-expressed with CAR-GPC3 (Supplementary Fig. S1a). The expression of CARs in the transduced T cells was measured by fluorescence-activated cell sorting analysis (FACS), and the CAR expression detection results are shown in Supplementary Fig. S1b. Patients received the lymphodepletion therapy regimen 6 days before CT017 CAR-GPC3 T-cell infusion, comprising cyclophosphamide (300 mg/m2) and fludarabine (25 mg/m2) for 3 days.
A 3 + 3 clinical trial design was applied (Supplementary Fig. S2). Patients were treated in a dose escalation/de-escalation scheme with a starting dose of 250 × 106 CAR T cells, and subsequent dose groups could be escalated to 400 × 106 CAR T cells or de-escalated to 150 × 106 CAR T cells per safety, preliminary efficacy, and pharmacokinetics profile. Dose-limiting toxicity (DLT) was observed over a period of 28 days. Patients were eligible to receive the second and third dose at 12 and 24 weeks after the first dose infusion, if the first dose of treatment was considered tolerable and potentially beneficial. Additionally, the second or third dose of CAR T-cell therapy could be administered as a monotherapy or in combination with anti-HCC treatments. If three patients in a certain dose group completed the first cell infusion and no DLT was observed, an additional three patients were enrolled and started the combination therapy of CAR T cells with routine drugs for HCC. Combination therapy could be sorafenib (or other multi-kinase inhibitors), or in combination with programmed death-1/programmed death-ligand 1 (PD-1/PD-L1) monoclonal antibodies, or other treatments that may benefit subjects. The clinical rationale or decision on combination therapy will be based on the subjects’ previous treatments, responses and tolerance assessed by the investigator. Sorafenib and other multi-kinase inhibitors may promote anti-tumor immunity through synergizing the effects of CAR T cells as well as modulating the tumor microenvironment as reported.22,26,30 The reactivating exhausted immune response by immune checkpoint inhibitors may prolong CAR T cells functional persistence to make CAR T cells better play the role of tumor-targeting immune infiltration and highly specific antitumor immune response.31
Computed tomography (CT) with contrast was used to assess the tumor response to treatment 1 month following cell infusion according to RECIST version 1.1. Adverse events (AEs) were coded using the Medical Dictionary for Regulatory Activities (version 21.1) and graded based on the Common Terminology Criteria for Adverse Events version 5.0 (CTCAE 5.0). Cytokine release syndrome (CRS) was graded and managed according to the American Society of Transplantation and Cellular Therapy (ASTCT) CRS grading criteria.32 DLTs were defined as CT017 treatment-related elevated bilirubin or glutamic-pyruvic transaminase (ALT) or glutamic-oxalacetic transaminase (AST) of Grade ≥3 lasting for more than 14 days; CT017 CAR-GPC3 T cell-related Grade ≥3 CRS lasting for more than 7 days; immune effector cell-associated neurotoxicity syndrome (ICANS) of Grade 3 or more; other treatment-related nonhematologic AEs of Grade 3 or more that last for more than 7 days (with the exception of Grade 3 fever, laboratory abnormalities of no clinical significance, and Grade 3 fatigue); CT017 treatment-related ≥ Grade 4 hematologic toxicity (except lymphopenia and leukopenia) that could not be recovered to ≤ Grade 2 after 14 days of treatment; and CT017 treatment-related hemophagocytic lymphohistiocytosis (HLH).
Analysis of CAR-GPC3 DNA copy numbers and cytokine measurements
The blood samples were collected from patients before and at pre-specified time points after CT017 CAR T-cell infusion. A fully validated real-time fluorescent quantitative polymerase chain reaction (qPCR) assay was applied to determine the CT017 CAR-GPC3 DNA copy numbers in peripheral blood as described previously.33 The plasma concentrations of interleukin (IL)-6, IL-8, IL-10, tumor necrosis factor alpha (TNF-α), and interferon-gamma (IFN-γ) were measured using a cytometric bead array according to the manufacturer’s instructions (BD Biosciences; catalog numbers 558276, 558277, 558274, 560112, and 560111, respectively). The plasma concentrations of IL-2 and IL-15 were measured using electrochemiluminescence according to the manufacturer’s instructions (Meso Scale Discovery, K151QQD, and K151RDD). Transforming growth factor-β1 (TGF-β1) levels were measured using enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (R&D Systems; catalog numbers SB100B).
Anti-drug antibody and replication competent lentivirus
Anti-CAR-GPC3 antibodies (ADA) in peripheral blood were tested before and at 28 days following CT017 CAR T-cell infusion and afterward by a fully validated ligand binding assay. The replication competent lentivirus (RCL) was determined by a fully validated qPCR assay.
Ethics
The study was approved by the institutional ethics committee of the First Affiliated Hospital of Zhejiang University (Approval No. 12 of the Research Ethics Review Conference in 2019). Written informed consent was obtained from patients before enrollment in the study.
Statistical analyses
Descriptive statistics consisted of medians with ranges and means with SDs for continuous variables and counts and percentages for categorical variables. Progression-free survival (PFS) and overall survival (OS) were analyzed using the Kaplan–Meier method. All analyses were performed with SAS 9.4.
Role of the funding source
This study was funded by CARsgen Therapeutics Co., Ltd. The study funder provided study drugs and participated in study design, data collection, data analysis, data interpretation, and writing of the report.
Results
Patient characteristics
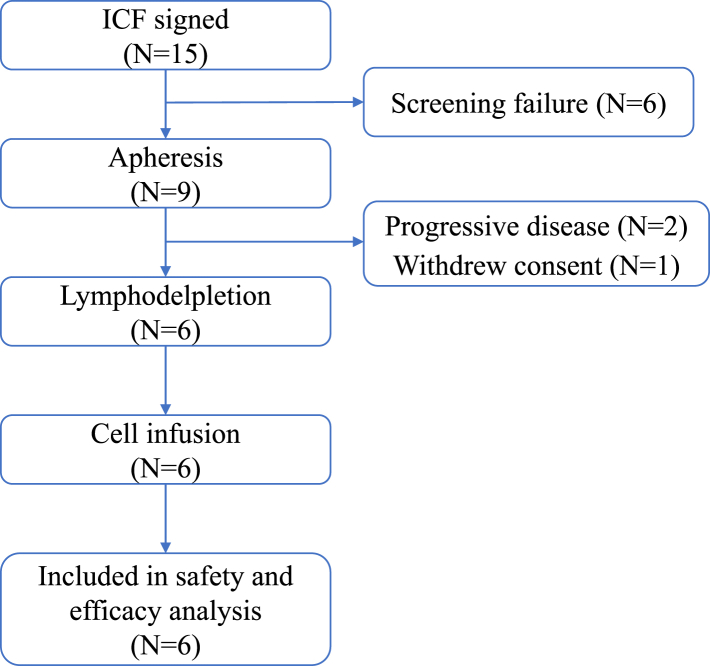
Between August 2019 and December 2020, 15 patients with GPC3-positive HCC signed the informed consent form after target screening, and nine patients underwent leukapheresis. As of the data cutoff date (29 August 2022), three of the nine patients discontinued the study before lymphodepletion, including two for rapid disease progression, and one due to patient decision. Six patients received a total of 7 infusions of CT017 CAR-GPC3 T cells at a dose level of 250 × 106 cells (Fig. 1, Supplementary Fig. S3).
Fig. 1.
Patient disposition. Abbreviations: ICF, informed consent forms; N, number.
Among the six patients who received cell infusion, the median age was 50.5 years (range, 36–66 years), and all patients were male. All patients had Barcelona Clinic Liver Cancer stage C disease and extrahepatic metastatic lesions. One (16.7%) patient had portal vein tumor thrombus, and four (66.7%) patients had clinically significant abnormal alpha-fetoprotein (AFP) levels. All six patients were diagnosed with HCC for a median time of 1.25 years and had received at least two prior lines of therapy. Among the six patients, four had received prior immunotherapy. Four patients had prior surgery and their primary tumors were removed, and the primary tumors of the other two patients were treated by transhepatic arterial chemoembolization (TACE). All patients had Child-Pugh class A disease with a median Child-Pugh score of 5. All patients had a history of hepatitis B, and five patients also had liver cirrhosis. All patients had an ECOG score of 1. GPC3 positivity was confirmed by immunohistochemistry. Of the six patients who received CT017, two were treated with CAR T-cell monotherapy and four in combination with regorafenib or sorafenib (one of whom started at the second infusion). No differences in baseline characteristics were observed between these two groups (Table 1).
Table 1.
Baseline characteristics.
| CT017 250 × 106 cells with combination therapy N = 4 |
CT017 250 × 106 cells without combination therapy N = 2 |
Total N = 6 |
|
|---|---|---|---|
| Median age (range), years | 50.5 (36–56) | 56.0 (46–66) | 50.5 (36–66) |
| Sex, n (%) | |||
| Male | 4 (100) | 2 (100) | 6 (100) |
| Female | 0 | 0 | 0 |
| ECOG score, n (%) | |||
| 0 | 0 | 0 | 0 |
| 1 | 4 (100) | 2 (100) | 6 (100) |
| Child-Pugh score | |||
| 5 | 4 (100) | 2 (100) | 6 (100) |
| Median duration of HCC (range), years | 1.30 (0.4–3.3) | 1.25 (1–1.5) | 1.25 (0.4–3.3) |
| Hepatitis B, n (%) | 4 (100) | 2 (100) | 6 (100) |
| AFP, n (%) | |||
| Normal | 2 (50.0) | 0 | 2 (33.3) |
| Abnormal, clinically significant | 2 (50.0) | 2 (100) | 4 (66.7) |
| Hepatocirrhosis, n (%) | |||
| Yes | 3 (75.0) | 2 (100) | 5 (83.3) |
| No | 1 (25.0) | 0 | 1 (16.7) |
| Disease type, n (%) | |||
| Hepatocellular carcinoma | 4 (100) | 2 (100) | 6 (100) |
| Pathological grading, n (%) | |||
| Moderately differentiated | 1 (25.0) | 1 (50.0) | 2 (33.3) |
| Poorly differentiated | 1 (25.0) | 0 | 1 (16.7) |
| Unknown/Other | 2 (50.0) | 1 (50.0) | 3 (50.0) |
| Portal vein tumor thrombus, n (%) | |||
| Yes | 1 (25.0) | 0 | 1 (16.7) |
| No | 3 (75.0) | 2 (100) | 5 (83.3) |
| Extrahepatic metastasis, n (%) | |||
| Yes | 4 (100) | 2 (100) | 6 (100) |
| No | 0 | 0 | 0 |
| BCLC stage, n (%) | |||
| Stage B: intermediate stage | 0 | 0 | 0 |
| Stage C: advanced stage | 4 (100) | 2 (100) | 6 (100) |
| GPC3 IHC staining intensity, n (%) | |||
| 1+ | 2 (50.0) | 0 | 2 (33.3) |
| 2+ | 0 | 0 | 0 |
| 3+ | 2 (50.0) | 2 (100) | 4 (66.7) |
| Previous lines, n (%) | |||
| 1 | 0 | 0 | 0 |
| 2 | 4 (100) | 2 (100) | 6 (100) |
| ≥3 | 0 | 0 | 0 |
| Previous surgery | |||
| Yes | 3 (75.0) | 1 (50.0) | 4 (66.7) |
| No | 1 (25.0) | 1 (50.0) | 2 (33.3) |
| Previous local therapies | |||
| TACE | 3 (75.0) | 2 (100.0) | 5 (83.3) |
| Radiotherapy | 0 | 1 (50.0) | 1 (16.7) |
| Previous systemic therapies, n (%) | |||
| Fluorouracil drugs | 1 (25.0) | 0 | 1 (16.7) |
| Platinum drugs | 1 (25.0) | 0 | 1 (16.7) |
| PD-1/PD-L1 inhibitor | 3 (75.0) | 1 (50.0) | 4 (66.7) |
| Targeted drugs | 4 (100) | 2 (100) | 6 (100) |
Abbreviations: AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; ECOG, Eastern Cooperative Oncology Group; GPC3, glypican-3; HCC, Hepatocellular carcinoma; IHC, immunohistochemistry; n, number in respective category; N, total number; PD-1, Programmed death-1; PD-L1, Programmed death-ligand 1; TACE, transcatheter arterial chemoembolization.
Characteristics of CT017 CAR-GPC3 T cells
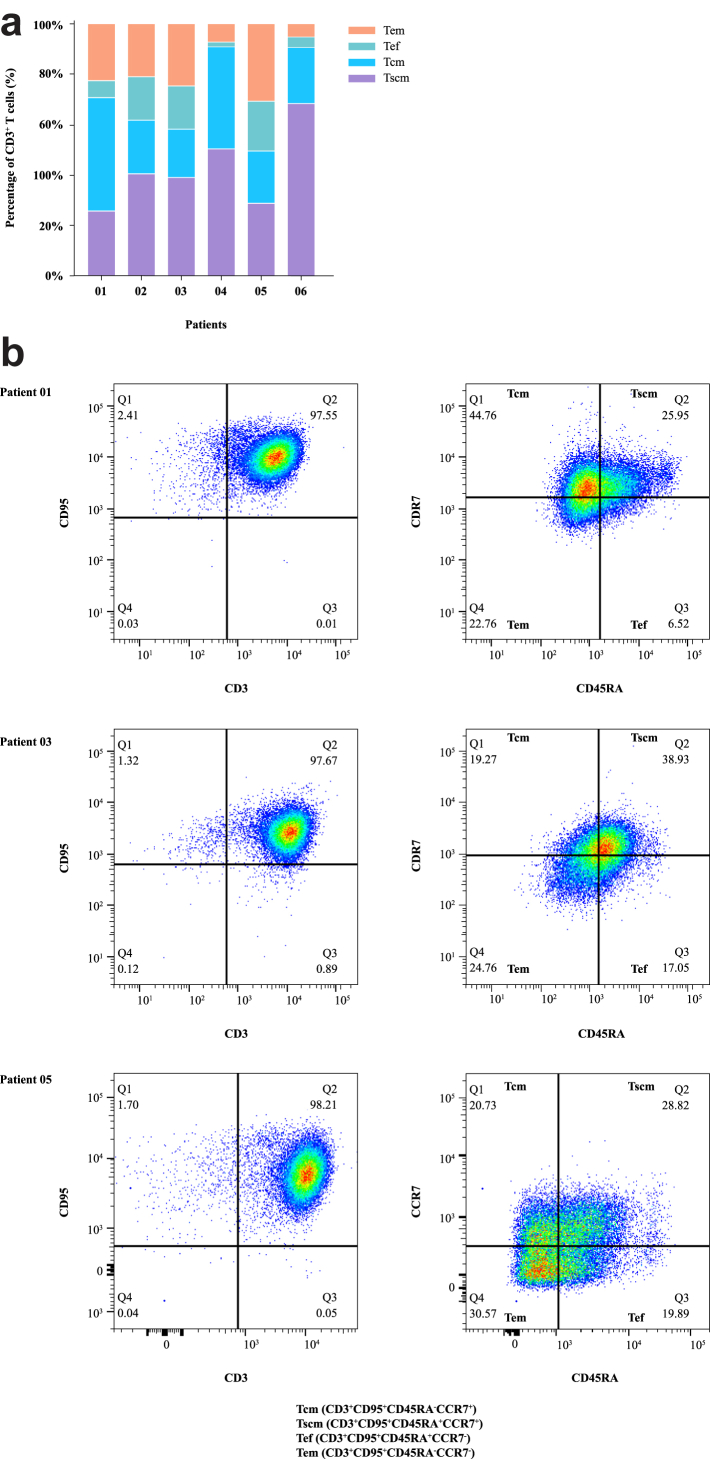
CT017 CAR-GPC3 T cells were successfully manufactured for all six patients. The median manufacturing time was 8 days (range 8–9). The median CD3+ T cells ratio was 98.6% (range 96.6%–99%), including helper T lymphocytes (Th) (52.9%, range 20.6%–84.6%) and cytotoxic T cells (CTL) (41.8%, range 9.5%–73.1%). Median transduction efficiency was 44.15% (range, 40.8%–46.2%). Further analysis showed that most of the T cells were stem cell-like memory T cells (Tscm) (39.1%, range 25.3%–64.8%), central memory T cells (Tcm) (21.2%, range 18.8%–43.7%), effector memory T cells (Tem) (21.6%, range 4.9%–30%), and effector T cells (Tef) (11.55%, range 2%–19.5%) (Fig. 2a). The representative FACS plots of the CAR T-cell phenotypes are shown in Fig. 2b.
Fig. 2.
The FACS results of the phenotype of CT017 CAR-GPC3 T cells. (a) The subset of CD3+ T cells of CT017 infused in each patient; (b) Representative FACS plots of CD3+CD95+ T cells (Patient 01, 03 and 05). Notes: FACS plots of CD3+CD95- T cells were not shown. Abbreviations: CD, cluster of differentiation; FACS, fluorescence-activated cell sorting; Tcm, central memory T cells; Tef, effector T cells; Tem, effector memory T cells; Tscm, stem cell-like memory T cells.
Study drug exposure
Six patients received a total of 7 infusions with a dosage of 250 × 106 cells, in which one patient received 2 infusions. The first three patients started with CT017 monotherapy, and patient 03 had SD after the first dose of CT017 CAR-GPC3 T cells and received a second infusion in combination with sorafenib decided by the investigator as the patient had been exposed to atezolizumab plus bevacizumab and lenvatinib. With no DLTs and significant safety signal identified, the next three patients received a combination treatment of CT017 CAR-GPC3 T cells and regorafenib with a dosage of 250 × 106 cells considering that the patients had been treated with PD-1/PD-L1 and/or no previous exposure with regorafenib. Dose escalation was not performed due to the investigator’s decision for safety concerns.
Safety
All patients had treatment-emergent adverse events (TEAEs), and 1 (16.7%) patient had a serious TEAE (anaphylactic shock). One patient discontinued from CT017 treatment due to AEs, and no patients withdrew from the study or died due to AEs. As shown in Table 2, hematologic toxicities were the most reported Grade 3 or 4 TEAEs, including lymphocyte count decreased (100%), platelet count decreased (66.7%), white blood cell count decreased (66.7%), neutrophil count decreased (50.0%). The hematologic TEAEs were expected toxicities of lymphodepletion chemotherapy and were manageable. The most common nonhematologic Grade 3 or 4 TEAEs included CRS (50.0%), blood fibrinogen decreased (33.3%), and pneumonia (33.3%).
Table 2.
Summary of all grade 3 or 4 TEAEs (Safety Analysis Set).
| Preferred term, n (%) | CT017 250 × 106 cells with combination therapy N = 4 |
CT017 250 × 106 cells without combination therapy N = 2 |
Total N = 6 |
|||
|---|---|---|---|---|---|---|
| Grade 3 or 4 | Any | Grade 3 or 4 | Any | Grade 3 or 4 | Any | |
| Any TEAE | 4 (100) | 4 (100) | 2 (100) | 2 (100) | 6 (100) | 6 (100) |
| Hematologic toxicities | ||||||
| Lymphocyte count decreased | 4 (100) | 4 (100) | 2 (100) | 2 (100) | 6 (100) | 6 (100) |
| Platelet count decreased | 3 (75.0) | 4 (100) | 1 (50.0) | 2 (100) | 4 (66.7) | 6 (100) |
| White blood cell count decreased | 2 (50.0) | 3 (75.0) | 2 (100) | 2 (100) | 4 (66.7) | 5 (83.3) |
| Neutrophil count decreased | 1 (25.0) | 2 (50.0) | 2 (100) | 2 (100) | 3 (50.0) | 4 (66.7) |
| Non-hematologic toxicities | ||||||
| Cytokine release syndrome | 2 (50.0) | 4 (100) | 1 (50.0) | 2 (100) | 3 (50.0) | 6 (100) |
| Pyrexia | 0 | 4 (100) | 1 (50.0) | 2 (100) | 1 (16.7) | 6 (100) |
| Hypoxia | 0 | 4 (100) | 1 (50.0) | 2 (100) | 1 (16.7) | 6 (100) |
| Blood fibrinogen decreased | 1 (25.0) | 1 (25.0) | 1 (50.0) | 2 (100) | 2 (33.3) | 3 (50.0) |
| Pneumonia | 1 (25.0) | 2 (50.0) | 1 (50.0) | 1 (50.0) | 2 (33.3) | 3 (50.0) |
| Hepatic function abnormal | 0 | 0 | 1 (50.0) | 2 (100) | 1 (16.7) | 2 (33.3) |
| Anaphylactic shock | 1 (25.0) | 1 (25.0) | 0 | 0 | 1 (16.7) | 1 (16.7) |
| Pulmonary oedema | 0 | 0 | 1 (50.0) | 1 (50.0) | 1 (16.7) | 1 (16.7) |
| Hypertension | 0 | 0 | 1 (50.0) | 1 (50.0) | 1 (16.7) | 1 (16.7) |
Abbreviations: AE, adverse event; CRS, cytokine release syndrome; N, number; TEAE, treatment-emergent adverse event.
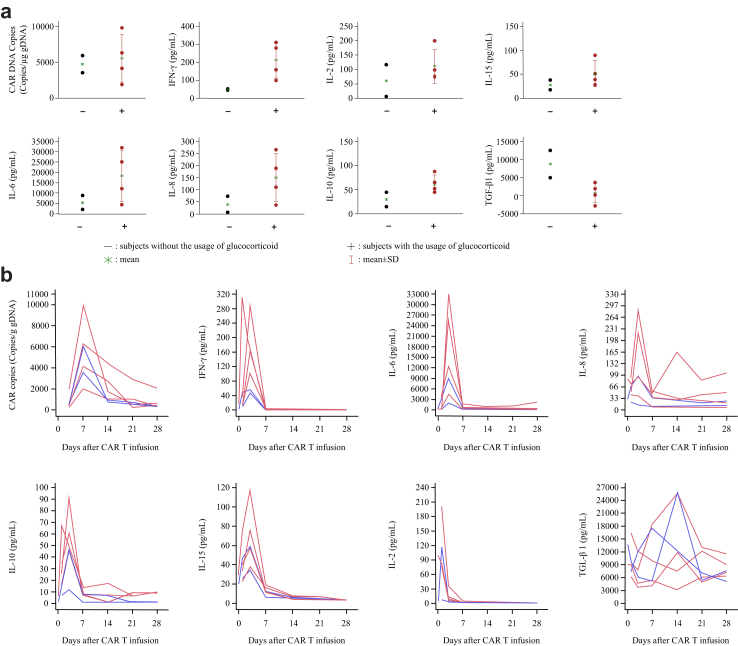
According to the ASTCT criteria, CRS occurred in all six patients with three at Grade 3. No neurotoxicity was identified. All patients received tocilizumab and four patients also received glucocorticoids for CRS. Patients receiving glucocorticoid treatment showed higher peak levels of IL-6, IL-8 and IFN-γ comparing with patients without glucocorticoids. However, patients with glucocorticoid treatment had lower TGF-β levels (Fig. 3a). For all patients, CRS started on the day of infusion, the median duration was 7 days (range 4–14 days), and cytokine concentrations aligned with the course of CRS (Fig. 3b).
Fig. 3.
CAR DNA copies and cytokines analysis post CT017 CAR-GPC3 T cells treatment. (a) Peak CAR DNA copies and the maximum change from baseline of cytokines concentration with/without the usage of glucocorticoid (safety analysis set); (b) Change of CAR DNA copies and cytokines after infusion of CT017 CAR-GPC3 T cells. Notes: The red color stands for the subjects with glucocorticoid, the blue color stands for the subjects without glucocorticoid. Abbreviations: CAR T, chimeric antigen receptor T cells; CAR, chimeric antigen receptor; IFN, interferon; IL, interleukin; TGF, transforming growth factor.
CT017 CAR-GPC3 T cells in peripheral blood and cytokine profile
CT017 CAR-GPC3 T cells were detectable in peripheral blood on the third day after infusion with a median copy number of 524.5 copies/μg gDNA (range 203–1913). The copy numbers increased rapidly, reaching a median peak of 5067 copies/μg gDNA (range 2001–9801) after 7 days, and the median area under the curve (AUC) for the CAR-GPC3 DNA copy numbers was 45,237.5 copies/μg gDNA (range 31,606–93,803) (Fig. 3b), indicating good expansion of the T cells. CT017 CAR-GPC3 T cells were still detectable 28 days after the first infusion in all patients with a median duration of 34 days (range 28–87). Patient 03 had a second infusion, with a lower peak copy number (2275 vs. 5962 copies/μg gDNA) compared with the first infusion. The patients with glucocorticoid treatment had similar peak copy numbers compared to patients without glucocorticoids (Fig. 3a).
Eight serum cytokines were monitored during the treatment. IL-2, IL-6, IL-8, IL-10, IL-15, and IFN-γ were elevated after the onset of CRS. IL-6 elevated dramatically and rapidly with a median peak value of 63,197.9 pg/mL (range 765.9–31,780) 3 days after infusion, and the median fold of increase in IL-6 level over baseline was 431.9 (range 36.3–1803.7). The median peak levels of each cytokine were as follows: IL-2 79.46 pg/mL (range, 7.12–199.54), IL-8 95.53 pg/mL (range, 21.76–280.91), IL-10 45.87 pg/mL (range, 11.71–88.44), IL-15 57.53 pg/mL (range, 34.74–115.56) and IFN-γ 100.52 pg/mL (range, 47.1–310.7).
Efficacy
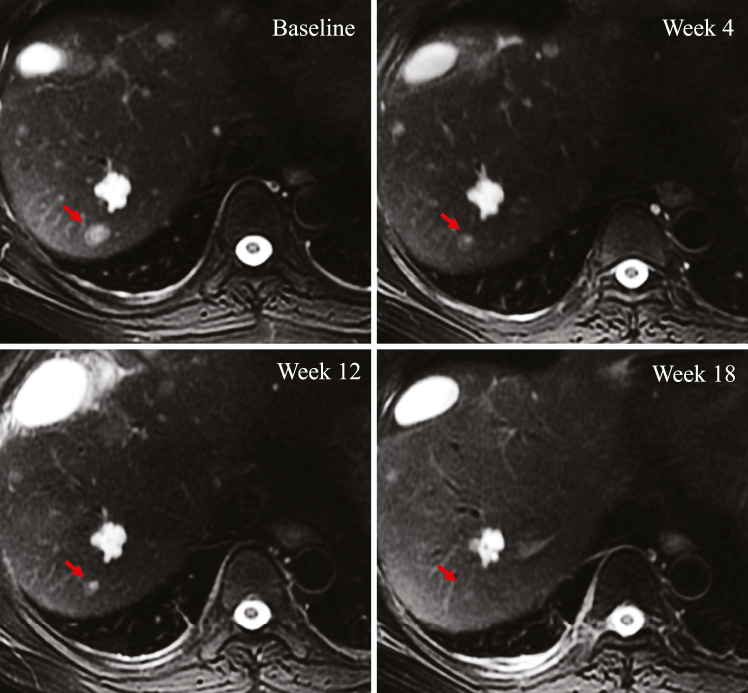
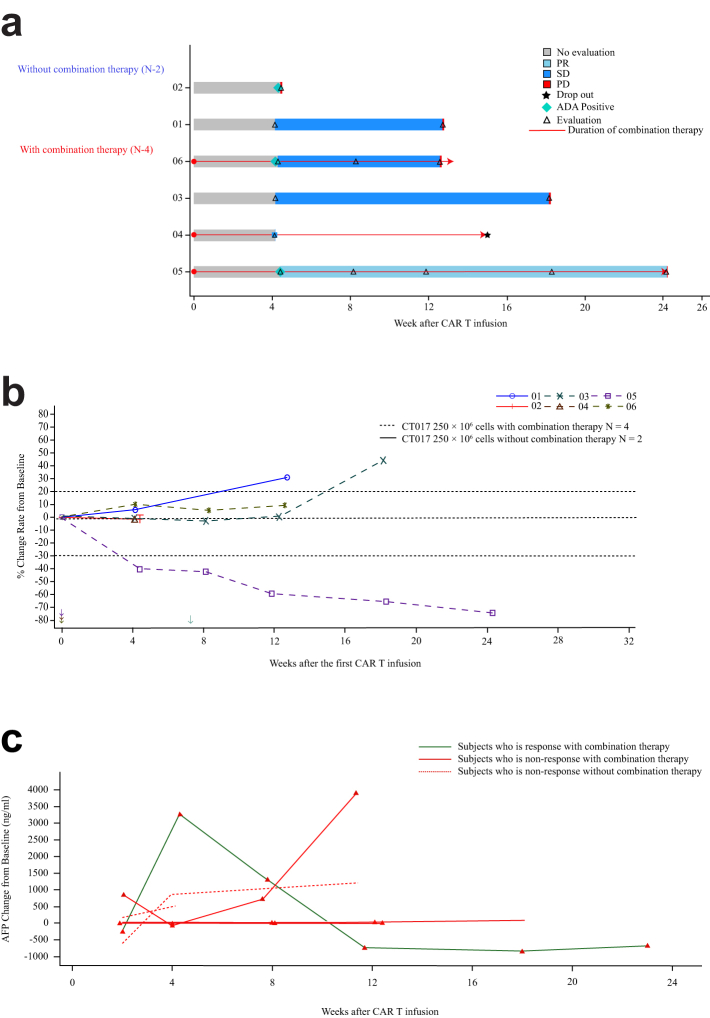
All six patients were evaluable for efficacy. One patient achieved a PR (Fig. 4), and the duration of response was 4.6 months. Two patients were evaluated as SD, one treated by CT017 CAR-GPC3 T-cell monotherapy while the other was treated in combination with regorafenib (Fig. 5a and b). The objective response rate was 16.7% and disease control rate was 50%. Changes in AFP expression were indicative of the efficacy of CAR T-cell therapy, where the treatment responders exhibited decreased AFP levels and non-responders showed stable or increased AFP levels (Fig. 5c).
Fig. 4.
The radiological imaging evaluation of target lesion of the patient with PR. Abbreviation: PR, partial response.
Fig. 5.
Clinical responses in patients with HCC administered CT017 CAR-GPC3 T cells. (a) Swimming plot of PFS (mITT analysis set); (b) Spider plot of tumor size change from baseline; Notes: Arrow marks the start time of combination therapy; (c) Relationship between Best overall response and change of AFP to baseline. Abbreviations: ADA, anti-drug antibodies; AFP, alpha-fetoprotein; CAR T, chimeric antigen receptor T cells; mITT, modified intention-to-treat analysis; PD, progressive disease; PR, partial response; SD, stable disease.
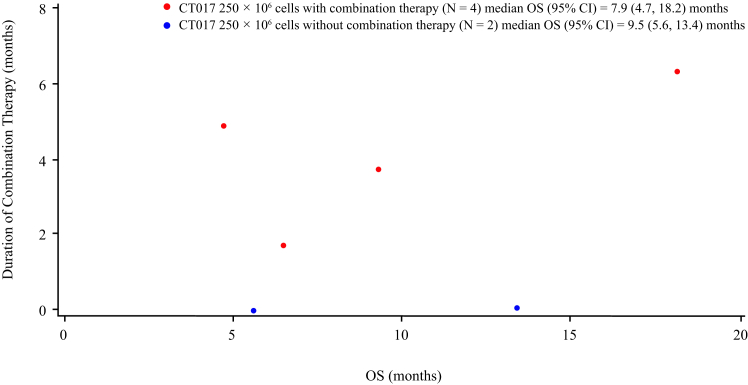
The median follow-up time was 7.87 months. The median PFS was 3.5 months (95% CI 0.9–4.9) and median OS was 7.9 months. Four patients treated with combination therapy, the duration of combination therapy is likely to be correlated with OS (Fig. 6); The median duration of disease control of one PR and two SD patients was 3.2 months [min 1.9, max 4.6]; however, due to the small sample size, the correlation should be further studied.
Fig. 6.
Scatter plot of duration of combination therapy and OS (mITT Analysis Set). Abbreviations: CI, confidence interval; mITT, modified intention-to-treat analysis; OS, overall survival.
Anti-drug antibody and replication competent lentivirus
Five patients underwent ADA testing. Two patients were negative, and the other three had positive ADA after CAR T-cell infusion (Supplementary Table S2). The ADA titer varied in patients over time, e.g., the titer for Patient 05 was 1 on D28 after infusion and increased to 125 on D128. In contrast, the ADA titer in Patient 06 was 56 and decreased to 12 on day 84. Patient 02 also had a positive ADA titer of 28 on D28. Five patients were tested for RCL, and all patients were negative.
Discussion
This was the first-in-human clinical trial of the fourth-generation CAR T cells targeting GPC3. CRS occurred in all patients, with 50% experiencing Grade 3 CRS. For all patients, CRS events started rapidly on the day of infusion. Compared with data in previous trial of Y035 CAR-GPC3 T-cell therapy,25 patients in this trial had a lower dose of infused CAR+ cells (250 × 106 cells vs. 700∼9250 × 106 cells) but higher incidence rate of CRS (100% vs. 69.2%), suggesting a potentially increased toxicity for this product; however, preclinical studies showed that cytokine levels in CAR-GPC3 T cells co-expressing RUNX3 are comparable to those in conventional CAR-GPC3 T cells.29 Accordingly, CAR DNA copy numbers in the peripheral blood after CT017 CAR-GPC3 T-cell infusion were analyzed, and the higher peak copy numbers were observed when compared with that in the clinical trial of conventional CAR-GPC3 T cells (5295 copies/μg gDNA vs. 360.4 copies/μg gDNA).25 It’s worth to be noted that despite the high incidence rate of CRS, no Grade 4 or 5 CRS occurred. None of the patients required admission to the intensive care unit. All patients recovered from CRS after treatment of tocilizumab with or without glucocorticoids. No DLT was identified, indicating that the therapy was generally manageable in late-line HCC patients who are at high unmet medical needs since no other alternative therapies are available. Notably, Patient 03 received two infusions, suggesting the feasibility of repeat infusion. In four patients treated with combination therapy, no increase in CRS incidence and severity was identified, with two patients (50.0%) having Grade 3 CRS, same as that in the patients who received CT017 monotherapy (one of two patients occurred with Grade 3 CRS). The study result showed that the combination of CT017 with TKI did not increase safety risk compared with that in the monotherapy group. This is in line with our previously published case report of a patient achieving long-term complete response with a manageable safety profile when administered with the combination of conventional GPC3-targeted CAR T therapy with sorafenib.26 With the presented four cases in this trial, more evidence of the feasibility of using the combination of TKI with CAR-GPC3 T to treat patients with advanced-stage HCC was obtained. Taken together, CT017 exhibited a manageable safety profile with a dose of 250 × 106 cells in monotherapy and in combination with TKI. To well manage the potential safety risk, close monitoring of cytokine levels during CAR T-cell therapy and early adequate doses of tocilizumab with or without steroids are recommended to mitigate further severe adverse events occurrence.
In the current study, one PR was observed in the combination group and no PR was found in the monotherapy group, suggesting that the addition of regorafenib may enhance the efficacy of CT017 CAR GPC3 T-cell therapy. Regorafenib is an oral multikinase inhibitor and is widely used for the treatment of advanced HCC. Previous studies have reported that regorafenib has immune-modulatory effects.34,35 A preclinical study has demonstrated synergistic effects of regorafenib and CAR-NK-92 cells in a mouse model with human colorectal cancer xenografts.36 Further studies evaluating the efficacy of combining regorafenib with CAR T-cell therapy are warranted due to limited evidence and lack of clear underlying mechanisms.
Four of the six patients had received prior anti-PD-1/PD-L1 immunotherapy. Among patients who had undergone prior immunotherapy, two achieved SD and one achieved PR post CT017 CAR-GPC3 T-cell therapy, while among patients without prior immunotherapy, none benefited from CT017 infusion. Based on these data, patients with prior use of immunotherapy may derive greater benefit from CT017 CAR-GPC3 T-cell therapy. These findings need to be further investigated in future studies.
With the median follow-up of 7.87 months post infusion, the median OS and median PFS were 7.9 months and 3.5 months in the heavily treated advanced HCC patients respectively. The longest survival time was 18.2 months without subsequent systemic treatment. Given that all patients enrolled in this trial had advanced-stage disease with high tumor burden and had failed extensive pretreatment (including immunotherapy), the survival outcome is promising which could be comparable to that in the patients at second line modality, including regorafenib (mPFS 3.1 and mOS 10.6 months), cabozantinib (mPFS 5.2 and mOS 10.2 months), ramucirumab (mPFS 2.8 and mOS 8.5 months), and apatinib (mPFS 4.5 and mOS 8.7 months).37 However, the sample size was too small to make a solid conclusion in the current situation.
In general, T cells are crucial for the anti-tumor activity of immunotherapy, but the mechanisms underlying their development and migration within tumors remain unknown. TRM cells are a subset of memory CD8+ T cells persisting in peripheral tissues.38 RUNX3 plays a key role in the retention of TRM cells in nonlymphoid tissues including solid tumors and promotes CD8+ T-cell recruitment.28 Based on the hypothesis that RUNX3 enhances the infiltration of CAR T cells into the tumor microenvironment, CT017 CAR-GPC3 T cells were designed. To our knowledge, this is the first clinical trial to investigate CAR T cells co-expression with RUNX3 in patients. The study observed a high frequency of CRS combined with IL-6 elevation and long duration of CAR T cells. However, since no liver biopsies were performed after CAR T-cell therapy, the evaluation of CAR T-cell infiltration in the tumor microenvironment remains unknown.
This trial was conducted at a single center in China where only Asian patients were enrolled. The sample size is small and the patients had different pathological grading status and experienced various prior therapies. Therefore, further studies with a larger sample size are warranted to explore the safety and efficacy in a more specified patient population who may be beneficial from the current investigational product.
Four patients in this trial were tested with GPC3 positive in a high percentage of tumor cells. Among them, only one patient with GPC3 positive in more than 80% of tumor cells achieved PR. Meanwhile, the other two patients with GPC3 positive in 20% tumor cells achieved SD. It seems that no clear relationship between the GPC3 staining area and clinical efficacy could be established, while it’s worth conducting further studies to well understand and find out what kind of disease characteristics may matter in the effectiveness of CAR-GPC3 T cells.
In summary, our study results preliminarily indicate that CT017 combination therapy may exhibit encouraging anti-tumor effects with manageable toxicities. The potential of CT017 CAR-GPC3 T cell therapy becoming a novel treatment modality for patients with advanced HCC is expected. Further investigation in larger trials is warranted to determine the advantages of combination therapy as well as the distinctive patient population with optimal risk/benefit profiles.
Contributors
The authors contributed to: conceptualization (QF, ZL and TL); data curation (YZ, WF and QZ); formal analysis (PZ, LL and YZ); funding acquisition (ZL and TL); investigation (QF, ZT, HZ and ML); methodology (QF, XZ and HW); project administration (YW and ZL); resources (YZ and DY); supervision (ZL, TL and XB); validation (WG); visualization (XD); writing—original draft (QF and YZ); and writing—reviewing and editing (QF, ZL and TL). All authors had full access to all the data and verified the data presented and accept responsibility to submit for publication. All authors read and approved the final version of the manuscript.
Data sharing statement
External researchers can make written requests to the corresponding author for sharing of data. Requests will be assessed on a case-by-case basis in consultation with lead and coinvestigators. A brief analysis plan and data request will be required and reviewed by the investigators for the approval of data sharing. When requests are approved, data will be sent electronically in password-protected files. All data sharing will abide by rules and policies defined by the sponsor, relevant institutional review boards, and local, state, and federal laws and regulations. Data sharing mechanisms will ensure that the rights and privacy of individuals participating in research will be guaranteed.
Declaration of interests
Zonghai Li, Huamao Wang, Yumeng Wang, Zhen Liu, Daijing Yuan and Wanwan Gao are employees of CARsgen Therapeutics Ltd., Shanghai, China. All the other authors declare no competing interests.
Acknowledgements
We thank the patients and their families and caregivers for participating in the study. This study was funded by CARsgen Therapeutics Co., Ltd. We acknowledge Wei Kan for her support with statistical output generation as well as Shumin Wang and Amanda Y. Hendrix for their support with manuscript revision.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102175.
Contributor Information
Zonghai Li, Email: zonghaili@carsgen.com.
Tingbo Liang, Email: liangtingbo@zju.edu.cn.
Appendix A. Supplementary data
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Llovet J.M., Ricci S., Mazzaferro V., et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 3.Kudo M., Finn R.S., Qin S., et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J., Qin S., Merle P., et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 5.Santoni M., Rizzo A., Kucharz J., et al. Complete remissions following immunotherapy or immuno-oncology combinations in cancer patients: the MOUSEION-03 meta-analysis. Cancer Immunol Immunother. 2023;72(6):1365–1379. doi: 10.1007/s00262-022-03349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abou-Alfa G.K., Lau G., Kudo M., et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022;1(8) doi: 10.1056/EVIDoa2100070. [DOI] [PubMed] [Google Scholar]
- 7.Qin S., Chan L.S., Gu S., et al. LBA35 camrelizumab (C) plus rivoceranib (R) vs. sorafenib (S) as first-line therapy for unresectable hepatocellular carcinoma (uHCC): a randomized, phase III trial. Ann Oncol. 2022;33:S1401–S1402. [Google Scholar]
- 8.Huang X., Xu L., Ma T., et al. Lenvatinib plus immune checkpoint inhibitors improve survival in advanced hepatocellular carcinoma: a retrospective study. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.751159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finn R.S., Qin S., Ikeda M., et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 10.Rizzo A., Cusmai A., Gadaleta-Caldarola G., Palmiotti G. Which role for predictors of response to immune checkpoint inhibitors in hepatocellular carcinoma? Expet Rev Gastroenterol Hepatol. 2022;16(4):333–339. doi: 10.1080/17474124.2022.2064273. [DOI] [PubMed] [Google Scholar]
- 11.Rizzo A., Ricci A.D., Di Federico A., et al. Predictive biomarkers for checkpoint inhibitor-based immunotherapy in hepatocellular carcinoma: where do we stand? Front Oncol. 2021;11 doi: 10.3389/fonc.2021.803133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Federico A., Rizzo A., Carloni R., et al. Atezolizumab-bevacizumab plus Y-90 TARE for the treatment of hepatocellular carcinoma: preclinical rationale and ongoing clinical trials. Expert Opin Investig Drugs. 2022;31(4):361–369. doi: 10.1080/13543784.2022.2009455. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Z., Tao C., Li J., Tang J.C.-O., Chan A.S.-C., Zhou Y. Chimeric antigen receptor T cells applied to solid tumors. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.984864. https://www.frontiersin.org/articles/10.3389/fimmu.2022.984864 [cited 2023 March 21]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mei H., Li C., Jiang H., et al. A bispecific CAR-T cell therapy targeting BCMA and CD38 in relapsed or refractory multiple myeloma. J Hematol Oncol. 2021;14(1):161. doi: 10.1186/s13045-021-01170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brudno J.N., Maric I., Hartman S.D., et al. T cells genetically modified to express an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of poor-prognosis relapsed multiple myeloma. J Clin Oncol. 2018;36(22):2267–2280. doi: 10.1200/JCO.2018.77.8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haas A.R., Tanyi J.L., O’Hara M.H., et al. Phase I study of lentiviral-transduced chimeric antigen receptor-modified T cells recognizing mesothelin in advanced solid cancers. Mol Ther. 2019;27(11):1919–1929. doi: 10.1016/j.ymthe.2019.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katz S.C., Moody A.E., Guha P., et al. HITM-SURE: hepatic immunotherapy for metastases phase Ib anti-CEA CAR-T study utilizing pressure enabled drug delivery. J Immunother Cancer. 2020;8(2) doi: 10.1136/jitc-2020-001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu N., Palmer D.C., Robeson A.C., et al. STING agonist promotes CAR T cell trafficking and persistence in breast cancer. J Exp Med. 2021;218(2) doi: 10.1084/jem.20200844. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7780733/ [cited 2023 February 23]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirabayashi K., Du H., Xu Y., et al. Dual targeting CAR-T cells with optimal costimulation and metabolic fitness enhance antitumor activity and prevent escape in solid tumors. Nat Cancer. 2021;2(9):904–918. doi: 10.1038/s43018-021-00244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dai H., Tong C., Shi D., et al. Efficacy and biomarker analysis of CD133-directed CAR T cells in advanced hepatocellular carcinoma: a single-arm, open-label, phase II trial. OncoImmunology. 2020;9(1) doi: 10.1080/2162402X.2020.1846926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pang N., Shi J., Qin L., et al. IL-7 and CCL19-secreting CAR-T cell therapy for tumors with positive glypican-3 or mesothelin. J Hematol Oncol. 2021;14(1):118. doi: 10.1186/s13045-021-01128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu X., Luo H., Shi B., et al. Combined antitumor effects of sorafenib and GPC3-CAR T cells in mouse models of hepatocellular carcinoma. Mol Ther. 2019;27(8):1483–1494. doi: 10.1016/j.ymthe.2019.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo M., Zhang H., Zheng J., Liu Y. Glypican-3: a new target for diagnosis and treatment of hepatocellular carcinoma. J Cancer. 2020;11(8):2008–2021. doi: 10.7150/jca.39972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ning S., Bin C., Na H., et al. Glypican-3, a novel prognostic marker of hepatocellular cancer, is related with postoperative metastasis and recurrence in hepatocellular cancer patients. Mol Biol Rep. 2012;39(1):351–357. doi: 10.1007/s11033-011-0745-y. [DOI] [PubMed] [Google Scholar]
- 25.Shi D., Shi Y., Kaseb A.O., et al. Chimeric antigen receptor-glypican-3 T-cell therapy for advanced hepatocellular carcinoma: results of phase I trials. Clin Cancer Res. 2020;26(15):3979–3989. doi: 10.1158/1078-0432.CCR-19-3259. [DOI] [PubMed] [Google Scholar]
- 26.Sun H., Xing C., Jiang S., et al. Long term complete response of advanced hepatocellular carcinoma to glypican-3 specific chimeric antigen receptor T-cells plus sorafenib, a case report. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.963031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milner J.J., Toma C., Yu B., et al. Runx3 programs CD8+ T cell residency in non-lymphoid tissues and tumours. Nature. 2017;552(7684):253–257. doi: 10.1038/nature24993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song Q., Shang J., Zhang C., Chen J., Zhang L., Wu X. Transcription factor RUNX3 promotes CD8+ T cell recruitment by CCL3 and CCL20 in lung adenocarcinoma immune microenvironment. J Cell Biochem. 2020;121(5–6):3208–3220. doi: 10.1002/jcb.29587. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y., Zhang H., Du G., et al. Enforced expression of Runx3 improved CAR-T cell potency in solid tumor via enhancing resistance to activation-induced cell death. Mol Ther. 2023;31(3):701–714. doi: 10.1016/j.ymthe.2022.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin Y.Y., Tan C.T., Chen C.W., Ou D.L., Cheng A.L., Hsu C. Immunomodulatory effects of current targeted therapies on hepatocellular carcinoma: implication for the future of immunotherapy. Semin Liver Dis. 2018;38(4):379–388. doi: 10.1055/s-0038-1673621. [DOI] [PubMed] [Google Scholar]
- 31.Grosser R., Cherkassky L., Chintala N., Adusumilli P.S. Combination immunotherapy with CAR T cells and checkpoint blockade for the treatment of solid tumors. Cancer Cell. 2019;36(5):471–482. doi: 10.1016/j.ccell.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee D.W., Santomasso B.D., Locke F.L., et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–638. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y., Di S., Shi B., et al. Armored inducible expression of IL-12 enhances antitumor activity of glypican-3-targeted chimeric antigen receptor-engineered T cells in hepatocellular carcinoma. J Immunol. 2019;203(1):198–207. doi: 10.4049/jimmunol.1800033. [DOI] [PubMed] [Google Scholar]
- 34.Ou D.L., Chen C.W., Hsu C.L., et al. Regorafenib enhances antitumor immunity via inhibition of p38 kinase/Creb1/Klf4 axis in tumor-associated macrophages. J Immunother Cancer. 2021;9(3) doi: 10.1136/jitc-2020-001657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu R.Y., Kong P.F., Xia L.P., et al. Regorafenib promotes antitumor immunity via inhibiting PD-L1 and IDO1 expression in melanoma. Clin Cancer Res. 2019;25(14):4530–4541. doi: 10.1158/1078-0432.CCR-18-2840. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Q., Zhang H., Ding J., et al. Combination therapy with EpCAM-CAR-NK-92 cells and regorafenib against human colorectal cancer models. J Immunol Res. 2018;2018 doi: 10.1155/2018/4263520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guidelines detail. NCCN. https://www.nccn.org/guidelines/guidelines-detail [cited 2023 June 8]. Available from:
- 38.Mami-Chouaib F., Blanc C., Corgnac S., et al. Resident memory T cells, critical components in tumor immunology. J Immunother Cancer. 2018;6(1):87. doi: 10.1186/s40425-018-0399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.