Abstract
Faciometrics have widely been used in contemporary studies on gender-related behavioral traits, for example, perceived and actual aggression, co-operation and trustworthiness, prejudicial beliefs, unethical behavior, and achievement drive, as well as, but to a lesser degree, in nonhuman primates. For the large part, these studies have focused primarily on “student-aged” populations with little empirical scrutiny regarding the efficacy of applying these measures with older participants. This study therefore investigated sexual dimorphism across four age-groups (20s, 30s, 40s, and 50s) in 444 participants (225 men). The expected sexual dimorphism was seen in the youngest age group in three of the four indices. The facial width to height ratio, however, although most commonly used empirically, was not found to be significantly different between men and women, consistent with more recent literature. Importantly, as age increased, sexual dimorphism decreased, but this was not consistent across all measures of it. Rather, it is evident that differing measures of sexual dimorphism follow distinct developmental trajectories. The only single marker which remained significantly different across all age-groups was cheekbone prominence. Sexual dimorphic faciometrics are therefore dynamic, declining, and differentiated through adulthood. Consequently, it is concluded that care should be taken in using faciometrics in studies involving older populations and that more research is needed to understand the impact of these distinct faciometric trajectories in gender- and masculinity-related studies.
Keywords: faciometrics, facial width to height ratio, fWHR, sexual dimorphism, aging, cheekbone prominence, life span
Faciometrics have widely been used in contemporary studies on gender- or dominance-related behavioral traits in both human and nonhuman primates. For example, faciometrics have been used in the empirical study of actual aggression (see Haselhuhn, Ormiston, & Wong, 2015, for a meta-analysis) and judgments of aggression (Carré, J. M., Morrissey, M. D., Mondloch, C. J., & McCormick, C. M, 2010), (Geniole, Molnar, Carré, & McCormick, 2014), co-operation and trustworthiness (Stirrat & Perrett, 2010, 2012), prejudicial beliefs (Hehman, Leitner, Deegan, & Gaertner, 2013), unethical behavior (Haselhuhn & Wong, 2011), and achievement drive (Lewis, Lefèvre, & Bates, 2012). Additionally, faciometrics have been employed in the study of brown capuchin monkeys (Sapajus apella) with regard to alpha status and assertive personality (Lefèvre et al., 2014) and on 22 species of the genus Macaca with regard to phenotypic markers of female dominance style (Borgi & Majolo, 2016). Faciometrics have also been utilized to understand the impact of static versus dynamic facial cues on the consistency of social evaluations (Hehman, Flake, & Freeman, 2015) as well as aging associated social heuristics (Hehman, Leitner, & Freeman, 2014), facial attractiveness (Danel & Pawlowski, 2007; Frackiewicz, 2001; Penton-Voak et al., 2001), sexual orientation (Hughes & Bremme, 2011; Valentova, Kleisner, Havlicek, & Neustupa, 2014), and the biological mechanisms underlying associations between anatomy and behavior (Lefèvre, Lewis, Perrett, & Penke, 2013; Pound, Penton-Voak, & Surridge, 2009). The majority of these studies have proposed that sexually dimorphic facial features may serve as a proxy for masculinity and thus enable us to understand how masculinity may be related to other behavioral traits. Importantly, however, and as Hehman, Leitner, and Freeman (2014) observe, there has been a dearth of research into the impact of aging on sexually dimorphic traits, with the implication that facial cues remain static over time.
Current Faciometric Indices
In many of these studies, the bizygomatic width (or the width between the left and right zygions or cheekbones) to upper face height (or the vertical distance between the midpoint of the upper lip to the nasion), otherwise known as the facial width to height ratio (fWHR), has been the measure of choice. However, despite the considerable evidence which supports associations between fWHR and gender- or dominance-related behavioral correlates (Borgi & Majolo, 2016; Carré & McCormick, 2008; Carré, McCormick, & Mondloch, 2009; Geniole et al., 2014; Haselhuhn et al., 2015; Haselhuhn & Wong, 2011; Lefèvre & Lewis, 2013; Lewis et al., 2012; Stirrat & Perrett, 2010), there is now increasing evidence that fWHR, should not be regarded as a proxy for sexual dimorphism. Indeed, though some research has suggested that the association between fWHR and these gender-related correlates may more correctly be attributed to a link with testosterone (see Lefèvre et al., 2013), more recent research also casts doubt on this relationship. For example, no evidence was found for a link between testosterone and fWHR in adult men, either when tested in a baseline measure or through three measures of competition-induced testosterone (Bird et al., 2016). Additionally, no link was found testosterone and fWHR in adolescent men, either at baseline or in testosterone-related traits (Hodges-Simeon, Hanson Sobraske, Samore, Gurven, & Gaulin, 2016). Moreover, further research supports greater fWHR in women over men (Hughes & Bremme, 2011; Little et al., 2008; Penton-Voak et al., 2001), whereas others, employing 2-D, 3-D, and anthropometric measures (Kramer, Jones, & Ward, 2012) or 2-D and 3-D metrics of homogenous groups differentiated by age and ethnicity (Lefèvre et al., 2012; Ozener, 2012), conclude that there is no evidence for sexual dimorphism of fWHR (see, too, Kramer, 2015; Lefèvre et al., 2013).
The most recent research in this area provides meta-analyses of the existing face research on fWHR, in addition to meta-analyses of the research on the fWHR of skulls only, on the premise that different explanations may be needed should sexual dimorphism be found in faces but not in skulls. It was concluded that though the meta-analyses of the skulls supported a larger fWHR in men than in women, this effect was small. It was also only true of East Asians once categorized by ethnicity and geographical origin. Additionally, reanalysis of the previous face research (i.e., excluding that research using skulls) suggested that, with the possible exception of East Asians, there is little support for sexual dimorphism in this facial metric (Kramer, 2017).
There are, nevertheless, many studies which have sought to investigate issues specifically related to sexual dimorphism via alternative measures. For example Penton-Voak et al. (2001), informed by Grammer and Thornhill (1994) and Scheib, Gangestad, and Thornhill (1999), introduced a composite masculinity index comprising facial width to lower face height, eye size, lower face height to face height, cheekbone prominence (ChP), and mean eyebrow height. Here, unlike previous studies, they explicitly reported on the levels of sexual dimorphism found, and in all measures, significant sexual dimorphism was shown. With regard to eye size and eyebrow height, both were significantly larger in women than in men. In terms, too, of the ratio-led faciometrics, ChP (measured by the horizontal distance between the most outward projecting points on the face at or below the eyes divided by the horizontal distance between left and right gonion approximations) was shown to be significantly greater in women than in men. Thus, a more feminine shape of face appears to be more heart shaped. With regard to facial width to lower face height (measured by the horizontal distance between the most outward projecting points on the face at or below the eyes divided by the vertical distance from the chin to the pupil), this, too, was shown to be larger in women than in men. Hence a shorter lower face relative to the midwidth is, again, more feminine. However, lower face height to full face height (LFH/FFH; as measured by the vertical distance from the chin to the pupil divided by the vertical distance from the chin to the hairline) is shown to be larger in men than in women. Here, then, a more feminine face is shorter in the lower area relative to total length. Such dimorphism has since been firmly established (Hughes & Bremme, 2011; Lefèvre et al., 2012, 2013; Little et al., 2008).
Others have utilized elements of the Penton-Voak et al. (2001) index. For example, Lefèvre et al. (2012), compared measures of lower face height to face height, ChP, and facial width to lower face height with measures of fWHR across four groups, whereas Little et al. (2008) also utilized three of these measures (lower face height to face height, ChP, and facial width to lower face height) but also introduced jaw height to lower face height. In addition, others have utilized all measures of the composite masculinity index as well as further possible indices of sexual dimorphism, namely, lip width to jaw width and forehead height (Hughes & Bremme, 2011). A less researched but alternative measure, the eye-mouth-eye angle, has also been found to be sexually dimorphic (Danel & Pawlowski, 2007; Frackiewicz, 2001), though this has received rather less empirical scrutiny.
Age and Faciometrics Indices
There is, then, a substantive literature based on facial metrics and sexual dimorphism, but issues regarding age and sexually dimorphic traits have been little addressed, with the majority of papers relying on the traditional student populations from which to draw samples. If, as Guégan,Teriokhin, and Thomas (2000) propose, sexual dimorphism is a response to selective pressures around fecundity, one might assume that sexual dimorphism will increase at puberty, peak over the period of greatest fertility, and decline thereafter. Anthropometric measures at puberty support this hypothesis (Greil, 2006) as do the sexually dimorphic regulatory mechanisms of growth hormones (Jaffe et al., 1998).
Such dimorphism may, of course, be explained by differences in underlying bone structure or by differences in musculature, the dermal layer, or in adiposity. For that reason, and as Hehman et al. (2014) discuss, as fWHR is based on underlying bone structure (as, too, are other facial dimensions more consistently accepted as sexually dimorphic), it may be erroneous to assume that the process of aging would have no bearing on these faciometrics. Certainly, with regard to underlying bone structure, it is known that while facial morphology changes most dramatically through childhood and adolescence, facial changes continue, albeit more slowly, throughout adult life. There may be increases in the heaviness of the face, for example, which may be at least partially explained by small amounts of continued appositional growth in the facial skeleton. Likewise in old age, the face becomes more shrunken in appearance as a result of some loss of bone mass (Atkinson, 2013).
Additionally, environmental stressors and the natural breakdown of collagen and elastin are both implicated in the degradation of the structure of the dermal layer (Yasui et al., 2013). So, too, is the hypoestrogenism caused by menopause which accelerates age-related deterioration of the skin, including, among other factors, a thinning of the skin, and reduction in elasticity (Stevenson & Thornton, 2007). Further, women have both higher total body fat as well as more subcutaneous body fat over their life span, than men (Greil, 2006), and the distribution of such tissue varies significantly between genders (Law, Bloor, Budge, & Symonds, 2014). As gender difference in adiposity is at least partly attributable to the effects of reproductive hormones, and as reproductive hormones change with age, it should be expected that age will impact levels of and distribution of adipose tissue (Yeung et al., 2013). Thus, surface proxies of underlying bone structure, as well as the underlying bone structure itself, may be liable to sexually dimorphic change. That surface proxies of, and underlying bone structure, may change dynamically over the life span suggests, therefore, that assumptions based on visible facial sexual dimorphism in a younger sample may not be usefully generalized to older adult samples.
Three studies known to us have addressed this issue. In a recent study designed specifically to consider the effects of life span changes to fWHR on social perceptions, Hehman et al. (2014) assessed male facial images of men drawn from two databases of images. Across the two samples (Study 1, Convict sample n = 387, Mage = 41.96, SD = 11.41, range = 20–77, and Study 2, nonconvict sample, n = 152, Mage = 48.83, SD = 15.4, representing each decade of life from 20–70s), it was found that there was a small but significant negative relationship between age and fWHR, and this was linear with time (i.e., it did not remain static for a period and then show a significant decline).
Lefèvre et al. (2012) considered the sexual dimorphism of fWHR in four adult samples, one of which was drawn from a population of 306 Caucasian adults aged 83 (137 men). In addition to fWHR, lower face to full face height, ChP, and facial width to lower face height were also assessed, as having been linked to reactive measures of testosterone (Pound et al., 2009), it allowed the researchers to use known sexually dimorphic measures to test their association with fWHR. No significant sexual dimorphism was found for fWHR (consistent with three of the four samples), but ChP and facial width/lower facial height were found to be sexually dimorphic in this elderly sample (i.e., greater in women than in men); and this was consistent with their three other samples. (Lower face height/face height was not reported for this sample for methodological reasons.)
Similarly, the most recent study, involving a large sample of Commonwealth Games athletes (Kramer, 2015), conducted correlations on both genders across five ethnic groups and found no relationship between age and fWHR in men, and although white women showed a negative fWHR/age correlation, Asian-oriental women showed a positive one. The research on age thus far is therefore clearly equivocal with respect to sexually dimorphic facial dimensions.
Study 1
In this study, we looked at the validity of four well-investigated, ratio-led, and purportedly sexually dimorphic measurements (although please see the literature review for discussion regarding fWHR) as detailed by Lefèvre et al. (2012) in order to establish whether a composite measure has more power to detect group differences than any single measure of sexual dimorphism and hence whether the use of a composite measure should be considered a prerequisite when investigating sexual dimorphism generally. In line with previous research, and based on the prediction that facial sexual dimorphism will diminish over age, the aim was to establish this initially within a more research typical, student-aged population.
Method
Materials
Following Hehman et al. (2015), facial photographs of 75 men and 75 women were collected from the MORPH longitudinal facial image database (Ricanek & Tesafaye, 2006) of 13,000 individuals and 55,000 facial photographs. (As photographs in the database were provided by adults specifically for research purposes, no further permissions were required from the ethics committee of the authors’ institution.) Images were selected by a research assistant naive to the research hypothesis but with an understanding of the selection criteria. Selection criteria required that all were aged 20–29 (men: mean age = 23.12, SD = 3.54, women: mean age = 23.09, SD = 3.63), thus representing a “student-aged” sample, were classified in the database as White European, none wore glasses, and all images selected were forward-facing and neutral in expression in accordance with established protocols. Individuals whose photographs did not permit accurate measurement (e.g., through hairstyle or garments) were excluded from further analysis. As there was no specific order to the database, the first images which met our criteria (and were classified as “White European” in the file descriptor) were chosen. The researcher then opened the image and assessed it against the remaining criteria, rejecting where necessary.
Facial measures
Facial measurements were taken by a research assistant naive to the nature of the study using the software ImageJ (Version 6), an open-source, Java-written program allowing analysis of scientific images. Using protocols established by Penton-Voak et al. (2001) as previously discussed, and following the faciometrics of the Lefèvre et al. (2012) study, the following faciometrics were investigated: (1) ChP (a/b), (2) face width to lower face height (a/c), (3) LFH/FFH (c/d), and (4) fWHR (a/e; see Figure 1). We mean by the horizontal distance (a) between right and left zygions and (b) between right and left gonions, and the vertical distance from (c) the nasion to the chin, (d) from the hairline to the chin, (e) from the nasion to the midpoint of the lips (see Figure 1).
Figure 1.
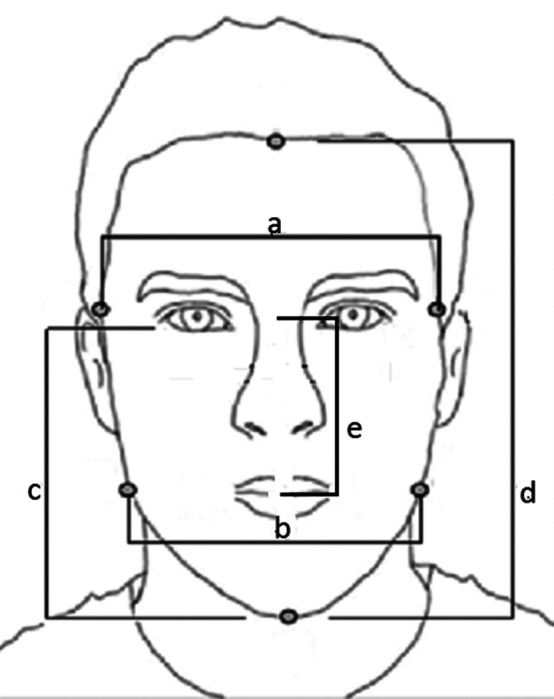
Points used in the calculation of facial metrics.
It should be noted, with respect to this final measure (e), that we depart slightly from previous measures in order to be as consistent as possible with skull allometry. For example, in terms of measuring the midpoint of the lips as opposed to the more usual upper point of the lips, we avoid individual differences in lip depth as the midpoint of the lips should fall reliably on the point where the teeth meet. By contrast, using the upper point of the lips may fall less reliably at a consistent skull position (i.e., the prosthion). There are, in fact, a number of protocols for this measure as observed in the meta-analysis by Haselhuhn, Ormiston, and Wong (2015). They concluded that the method of facial height measurement did not appear to unduly influence the overall results. However, in order to assess the potential impact of using our revised measure, we analyzed the impact of adopting this new measure against a previously adopted measure, that being the upper lip to the highest point of the eyelids. There was a strong positive correlation between the two (r = .99, N = 50, p > .001) and independent t-tests for gender revealed virtually identical results between the adopted measure, t(48) = 4.49, p > .001, r = .54, and the previously reported measure, t(49) = 4.41, p > .001, r = .54. Additionally, verifying that the new landmarks were easily identified, measurements taken by two independent researchers showed an interclass correlation of r = .98, N = 50, p > .001, indicating excellent interrater reliability.
Results
A one-way between groups multivariate analysis of variance (ANOVA) was conducted to investigate facial sexual dimorphism in a student-aged group. Four dependent variables were included: ChP, facial width to lower face height, LFH/FFH, and lastly, fWHR. There was a statistically significant difference between men and women on the combined dependent variables, F(4, 145) = 11.53, p < .001, partial η2 = .24. When the results for the dependent variables were then considered separately (and having made a Bonferroni adjustment of the α level to .0125, reflecting the four dependent variables), two of the four dependent variables retained statistical significance—ChP, F(1, 148) = 25.79, p < .001, partial η2 = .15, and facial width to lower facial height, F(1, 148) = 12.83, p < .001, partial η2 = .08. Although significant at the original .05 level, LFH/FFH narrowly failed to reach significance at the adjusted level, F(1, 148) = 4.56, p = .034, partial η2 = .03. fWHR was not significant. Descriptive statistics may be found in Table 1.
Table 1.
Main Effect for Sexual Dimorphism in Individual Facial Metrics.
| Age groups | M (SD) | 95% CI | F | |||
|---|---|---|---|---|---|---|
| Cheekbone prominence | 69.37*** | .15 | ||||
| 20sa*** | Male | 1.16 (.06) | [1.14, 1.18] | |||
| Female | 1.21 (.07) | [1.19, 1.23] | ||||
| 30sa** | Male | 1.14 (.07) | [1.12, 1.16] | |||
| Female | 1.19 (.08) | [1.17, 1.21] | ||||
| 40sa** | Male | 1.13 (.07) | [1.11, 1.15] | |||
| Female | 1.17 (.06) | [1.16, 1.19] | ||||
| 50sb*** | Male | 1.12 (.08) | [1.10, 1.14] | |||
| Female | 1.20 (.07) | [1.18, 1.22] | ||||
| Face width to lower face height | 21.70*** | .05 | ||||
| 20sa* | Male | 1.14 (.07) | [1.11, 1.17] | |||
| Female | 1.18 (.07) | [1.15, 1.21] | ||||
| 30sa** | Male | 1.18 (.11) | [1.03, 1.34] | |||
| Female | 1.26 (.14) | [1.23, 1.29] | ||||
| 40sa*** | Male | 1.18 (.11) | [1.15, 1.21] | |||
| Female | 1.27 (.13) | [1.24, 1.30] | ||||
| 50sb | Male | 1.22 (.11) | [1.19, 1.25] | |||
| Female | 1.22 (.10) | [1.19, 1.26] | ||||
| Lower face height to full face height | 2.18 | .01 | ||||
| 20sa** | Male | 0.67 (.03) | [0.65, 0.68] | |||
| Female | 0.65 (.04) | [0.63, 0.66] | ||||
| 30sa | Male | 0.61 (.06) | [0.59, 0.62] | |||
| Female | 0.60 (.06) | [0.58, 0.61] | ||||
| 40sa | Male | 0.61 (.06) | [0.59, 0.62] | |||
| Female | 0.59 (.06) | [0.58, 0.61] | ||||
| 50sb | Male | 0.60 (.05) | [0.59, 0.62] | |||
| Female | 0.61 (.06) | [0.60, 0.63] | ||||
| fWHR | .68 | .00 | ||||
| 20sa | Male | 2.15 (.26) | [1.88, 2.42] | |||
| Female | 2.17 (.42) | [1.90, 2.44] | ||||
| 30sa | Male | 2.01 (.24) | [1.74, 2.28] | |||
| Female | 2.06 (.31) | [1.79, 2.33] | ||||
| 40sa | Male | 1.98 (.17) | [1.71, 2.25] | |||
| Female | 2.03 (.14) | [1.76, 2.30] | ||||
| 50sb | Male | 2.39 (2.63) | [2.12, 2.66] | |||
| Female | 1.94 (.18) | [1.66, 2.23] | ||||
Note. CI = confidence interval; = partial η2; fWHR = facial width to height ratio.
an = 100 (50 men). bn = 94 (50 men).
*p < .05. **p < .01. ***p < .001.
Discussion
Study 1 provides support for the power of a composite of measures to detect sexual dimorphism in a student-aged sample, indicating a large effect size in the multivariate test statistic (partial η2; Cohen, 1988). Perhaps not surprisingly it was also evident that the composite measure had greater power to establish sexual dimorphism in this sample than any of the facial metrics considered independently. Nevertheless, ChP, facial width to lower face height, and LFH/FFH independently indicated large, medium, and small effect sizes, respectively. No significant sexual dimorphism, however, was seen in fWHR, with sex explaining only a negligible amount of its variance. This was consistent with those previous studies reporting no sexual dimorphism in this trait (Kramer, 2015; Kramer et al., 2012; Lefèvre et al., 2012; Lefèvre, Lewis, Perrett, & Penke, 2013; Ozener, 2012).
Study 2
In this study, we assessed sexual dimorphism across three further decades of life, these being the 30s, 40s, and 50s utilizing analysis of the individual sexually dimorphic traits in addition to the composite of these measures as established in Study 1. The aim was to understand how facial sexual dimorphism may be influenced by aging as well as to establish the efficacy of using such a composite over any individual measure of sexual dimorphism.
Method
Materials and facial measures
Following the same criteria as that in Study 1, facial photographs of a further 150 men and 144 women, drawn equally from each decade of life, were selected (i.e., from 30–39 to 50–59) in addition to the 150 male and female images from Study 1. (For mean age and SD of individuals in photographs, see Table 2.) Images were initially selected in order of presentation in the data set (as in Study 1), the first images in each age-group which conformed to the selection criteria as detailed in the Materials section of Study 1 being chosen. However, this resulted in significant differences in mean age between the sexes in each group, so the remaining 50% of images in each age-group were additionally screened to match for age with the opposite gender. By so doing, we were able to ensure no significant difference in mean age between the sexes in any group.
Table 2.
Mean Age (and SD) of Individuals by Gender and Age-Group.
| 20–29 | |||||
|---|---|---|---|---|---|
| Gender | Study 1 | Study 2 | 30–39 | 40–49 | 50–59 |
| Male | 23.12 (3.54) | 20.72 (0.88) | 34.22 (3.32) | 44.44 (3.77) | 53.72 (2.73) |
| Female | 23.09 (3.63) | 20.68 (1.25) | 35.36 (3.02) | 45.12 (2.86) | 54.00 (2.76) |
In order to address a violation of the assumption of homogeneity of variance–covariance matrices, it was also decided to reduce the number of images analyzed from the 20s age-group in order to create equal sample sizes, ensuring confidence that Pillai’s trace would be robust (Field, 2009; Hakstian, Roed, & Lind, 1979). The first 100 images were selected with the removal of the last 50.
Results
A bootstrapped two-way between-groups multivariate analyses of variance was first conducted to investigate facial sexual dimorphism across the four decades of life, that is, the 20s, 30s, 40s, and 50s, using the four facial metrics used in Study 1. There was a statistically significant interaction between gender and age-group, F(12, 1155) = 1.87, p = .035; Pillai’s trace = .06; partial η2 = .02. However, when the results for the facial metrics were considered independently, none were significant.
When looking at the main effect of sexual dimorphism, there was statistically significant sexual dimorphism in the combined facial metrics, F(4, 383) = 24.71, p < .001, Pillai’s trace = .21; partial η2 = .21. Independently, however (and having made the necessary Bonferroni adjustment to α level), the only facial metrics which were significantly sexually dimorphic were ChP, F(1, 386) = 69.37, p < .001, partial η2 = .15, and facial width to lower facial height, F(1, 386) = 21.70, p < .001, partial η2 = .05. Descriptive and inferential statistics for the sexual dimorphism of the individual facial metrics, reported for each age-group, are, again, indicated in Table 1.
Finally, when looking at the main effect of age-group, there was a statistically significant difference between age-groups in the combined dependent variables, F(12, 1155) = 6.97, p < .001; Pillai’s trace = .20; partial η2 = .07. Independently, the effect of age was significant for ChP, F(3, 386) = 4.18, p = .006, partial η2 = .03; facial width to lower facial height, F3, 386) = 8.93, p < .001, partial η2 = .07; lower facial height to full facial height, F(3, 386) = 26.40, p < .001, partial η2 = .17, but not for fWHR, F(3, 386) = 69.37, p = .544, partial η2 = .01. For ChP, facial width to lower facial height and lower facial height to full facial height the twenties age group was significantly different from all other age groups. However, none of the other age groups were significantly different from each-other. For fWHR there were no significant differences between any of the age groups. See Table 3.
Table 3.
Main Effect of Age-Group in Individual Facial Metrics.
| Age groups | Mean diff. | SE | 95% CI | F | |||
|---|---|---|---|---|---|---|---|
| Cheekbone prominence | 4.18** | .03 | |||||
| 20sa | 30sa | .02* | .01 | [.00, .04] | |||
| 40sa | .03** | .01 | [.01, .05] | ||||
| 50sb | .02* | .01 | [.00, .04] | ||||
| Face width to lower face height | 8.93*** | .07 | |||||
| 20sa | 30sa | −.06*** | .02 | [−.09, −.03] | |||
| 40sa | −.07*** | .02 | [−.10, −.04] | ||||
| 50s | −.06*** | .02 | [−.09, −.03] | ||||
| Lower face height to full face height | 26.40*** | .17 | |||||
| 20sa | 30sa | .05*** | .01 | [.04, .07] | |||
| 40sa | .06*** | .01 | [.04, .07] | ||||
| 50sb | .05*** | .01 | [.04, .06] | ||||
| fWHR | .72 | .01 | |||||
| 20sa | 30sa | .12 | .14 | [.15, .39] | |||
| 40sa | .16 | .14 | [−.12, .42] | ||||
| 50sb | −.01 | .14 | [−.28, .27] | ||||
Note. SE = standard error; CI = confidence interval; = partial η2; fWHR = facial width to height ratio.
an = 100 (50 men). bn = 94 (50 men).
*p < .05. **p < .01. ***p < .001.
A two-way between groups ANOVA was also conducted to investigate facial sexual dimorphism across the four decades of life in a composite measure of the combined facial metrics, including ChP, facial width to lower face height, LFH/FFH, and fWHR. There was no significant interaction between age-group and gender, F(3, 386) = 1.16, p = .324, partial η2 = .009, nor was there a significant effect for age, F(3, 386) = 1.38, p = .249, partial η2 = .01 (see Table 4). There was, however, a significant effect for gender, F(3, 386) = 4.02, p = .046, partial η2 = .01 (see Table 5).
Table 4.
Main Effect of Age-Group of Composite Analyses Including and Excluding fWHR.
| Age groups | Mean diff. | SE | 95% CI | F | ||
|---|---|---|---|---|---|---|
| Composite including fWHR | 1.38 | .01 | ||||
| 20sa | 30sa | .22 | .14 | [−.06, .49] | ||
| 40sa | .25 | .14 | [−.03, .52] | |||
| 50sb | .08 | .14 | [−.19, .35] | |||
| Composite excluding fWHR | 7.94*** | .12 | ||||
| 20sa** | 30sa | −.10 | .02 | [−.14, −.05] | ||
| 40sa | −.09 | .02 | [−.13, −.05] | |||
| 50sb | −.09 | .02 | [−.13, −.05] |
Note. SE = standard error; CI = confidence interval; = partial η2; fWHR = facial width to height ratio.
an = 100 (50 men). bn = 94 (50 men).
*p < .05. **p < .01. ***p < .001.
Table 5.
Main Effect of Sexual Dimorphism of Composite Analyses Including and Excluding fWHR.
| Age groups | M (SD) | 95% CI | F | |||
|---|---|---|---|---|---|---|
| Composite including fWHR | 4.02* | .01 | ||||
| 20sa | Male | .51 (0.26) | [0.44, 0.59] | |||
| Female | .43 (0.42) | [0.32, 0.56] | ||||
| 30sa | Male | .30 (0.32) | [0.22, 0.40] | |||
| Female | .21 (0.36) | [0.12, 0.32] | ||||
| 40sa* | Male | .27 (0.27) | [0.19, 0.35] | |||
| Female | .18 (0.21) | [0.12, 0.23] | ||||
| 50sb | Male | .65 (2.61) | [0.25, 1.42] | |||
| Female | .13 (0.19) | [0.07, 0.19] | ||||
| Composite excluding fWHR | 50.10*** | .12 | ||||
| 20sa*** | Male | 1.64 (0.09) | [1.62, 1.66] | |||
| Female | 1.73 (1.0) | [1.71, 1.77] | ||||
| 30sa*** | Male | 1.71 (0.17) | [1.67, 1.76] | |||
| Female | 1.85 (0.20) | [1.80, 1.91] | ||||
| 40sa*** | Male | 1.71 (0.18) | [1.65, 1.76] | |||
| Female | 1.85 (0.22) | [1.79, 1.91] | ||||
| 50sb* | Male | 1.74 (0.14) | [1.70, 1.78] | |||
| Female | 1.82 (0.15) | [1.77, 1.86] | ||||
Note. SD = standard deviation; CI = confidence interval; = partial η2; fWHR = facial width to height ratio.
an = 100 (50 men). bn = 94 (50 men).
*p < .05. **p <.01. ***p < .001.
Nevertheless, as fWHR was, as previously discussed, questionable for inclusion as a sexually dimorphic ratio, and as the independent analyses across all ages-groups corroborated that, a new composite measure was also calculated, this time excluding fWHR, and thereby offering a truer reflection of the combined impact of the facial metrics on sexual dimorphism between age-groups. A two-way between groups ANOVA showed, again, no significant interaction between age-group and gender, F(3, 386) = 1.04, p = .377, partial η2 = .008. There was, however, a significant effect for age, F(3, 386) = 7.94, p < .001, partial η2 = .06 (see Table 4). Additionally, there was a significant effect for gender, F(3, 386) = 50.10, p < .001, partial η2 = .12 (see Table 5). For this composite, the 20s age-group was significantly different from all other age-groups (p < .01). However, none of the other age-groups were significantly different from each other.
Discussion
The first aim of Study 2 was to establish the efficacy of using a composite of measures over any individual measure in progressively older age-groups. Consideration of the composite measure of the four facial metrics indicated no significant interaction between age and gender, and no effect for age, but a significant effect of gender with a small effect size. In other words, a composite measure including fWHR was just able to establish sexual dimorphism and suggests no impact of age on these facial metrics. By contrast, when considering each measure of sexual dimorphism as part of a multivariate analysis, significant sexual dimorphism was indicated with large effect size as well as a significant effect for age with moderate effect size.
We then decided to exclude fWHR as a valid facial metric (supported in the more recent literature and, indeed, through the nonsignificant dimorphism in every age-group in the current study) which allowed the opportunity to assess the efficacy of a revised composite of the three remaining facial metrics, thus offering a more parsimonious measure with improved validity. Consideration of this revised composite revealed, this time, a significant effect for both gender and age, both with moderate (i.e., increased) effect sizes and showing a general reduction in sexual dimorphism as age progresses. Thus, it is clear that a composite of ChP, lower face to full face height, and facial width to lower facial height is better able to explain the variance in sexual dimorphism over age than is a composite including fWHR.
Nevertheless, inspection of the multivariate analysis not only established significance for sexual dimorphism and age but additionally indicated a small but significant interaction between the two variables. This was explained by the impact of facial width to lower face height, with a changing trajectory for men and women in later middle age. It appears, therefore, that independent analysis is able to provide a more holistic understanding of the relationship between the variables and the individual trajectories of each over and above the use of the composite measure.
Overall, then, we can see that the sexually dimorphic facial metrics investigated most commonly in student-aged samples are not static over time, but rather there is a significant effect of aging on these facial metrics. Moreover, we can see that individual facial metrics do not follow the same trajectories over time. For example, although both sexually dimorphic, ChP remains significantly sexually dimorphic in every age–group, whereas the facial width to lower face height ratio is significantly sexually dimorphic in the 20s, 30s, and 40s, but not in the 50s. On the other hand, although sexually dimorphic in the 20s, the lower face to full face height ratio was not found to be significantly sexually dimorphic in any of the older age-groups. fWHR, on the other hand, was not significant in any age-group and also showed no change over time.
General Discussion
In the current studies, we initially investigated facial sexual dimorphism in adult humans through the use of a composite of known, sexually dimorphic facial measures in a student-aged population. We then investigated the stability of facial sexual dimorphism through middle adulthood in a sample of adults from three further decades of life. In the first study, we found that analysis of a composite of these measures showed significant differences between the sexes, with sex explaining a large amount of the variance in the composite measure. Independently, we found sex differences in three of the four measures tested (ChP, lower face to full face height, and facial width to lower facial height), consistent with prior research and expectations (Hughes & Bremme, 2011; Lefèvre et al., 2012; Little et al., 2008; Penton-Voak et al., 2001). We did not find sexual dimorphism of fWHR, further consolidating the growing consensus of opinion in that area (see Kramer, 2015; Kramer et al., 2012; Lefèvre et al., 2012; Lefèvre et al., 2013; Ozener, 2012). However, as fWHR may have contributed, through its correlations with the other measures, to the sexual dimorphism found in the composite, this was retained for analysis in the second study.
We then assessed three further groups for sexual dimorphism, representing the following three decades of life, as a composite and by the single measures of dimorphism. By using the composite, we were able to establish sexual dimorphism through every decade of middle adulthood. Additionally, and consistent with expectation, we were able to establish a reduction in sexual dimorphism over age. In other words, and as Hehman et al. (2014) found in their study on fWHR, facial sexual dimorphism cannot be accepted as static over time (though note, in the current study, sexual dimorphism was not found in fWHR in any age-group, including the “student-aged” sample). Inferences drawn, therefore, about behaviors linked to sexually dimorphic features in student-aged samples should not safely be assumed to generalize to older populations. However, future research on the influence of facial sexual dimorphism in middle age on behaviors, attitudes, or perceptions of others may usefully adopt this combined measure as a measure of discernible facial sexual dimorphism.
It was also evident, however, that the decline in dimorphism of the component parts of facial sex differences, just as in other areas of the body (Greil, 2006), does not follow the same temporal trajectory. Indeed, there appears to be considerable disparity between the various measures. For example, despite sex differences in lower facial height to full facial height being well supported within a student population (Hughes & Bremme, 2011; Lefèvre et al., 2012; Little et al., 2008; Penton-Voak et al., 2001), this dimorphism is shown here to be absent by early middle age. By contrast, ChP, again well supported within a student-aged population (Hughes & Bremme, 2011; Lefèvre et al., 2012; Little et al., 2008; Penton-Voak et al., 2001), appears not to lose any of its dimorphism from early adulthood through to late middle age. Indeed, the only other known study which investigates ChP in an aging population indicates that such dimorphism is still evident in the 80s (Lefèvre et al., 2012). We know that age-related changes in fWHR influence stereotypical social perceptions (Hehman et al., 2014). It is conceivable, too, that changes in specific sexually dimorphic measures may differentially impact social perceptions, and this is worthy of future investigation. In such studies, therefore, it would be beneficial to adopt a more differentiated approach to the measures of sexual dimorphism employed.
Exactly why some traits remain relatively static over time and others change may potentially be explained by the influence of circulating hormones on the dermal layer and adiposity. The changes in both seen over middle adulthood would be consistent with Guégan et al.’s (2000) thesis that sexual dimorphism is a response to selective pressures around fecundity. A limitation of the current study is that there was no knowledge of the reproductive position of the women photographed, many of whom in the older age-groups would be perimenopausal, menopausal, or postmenopausal. This period is, of course, marked by a reduction in estrogen levels which, as previously discussed, leads to both a thinning of the skin and a reduction in its elasticity (Stevenson & Thornton, 2007). Additionally, as estrogen and body fat are positively associated (Ziomkiewicz, Ellison, Lipson, Thune, & Jasienska, 2008) and as women have higher total body fat over their life span than men (Greil, 2006), the impact of hypoestrogenism would likely be greater where facial adiposity is greatest. Thus, differing adiposity both between the sexes and in individual facial distribution would logically result in differing trajectories as a result of hormonal changes.
It is also possible that changes to the underlying facial bone structure may differentially impact on the trajectories investigated. There is certainly evidence to suggest that contrary to traditional understanding, craniofacial morphology continues to change throughout adulthood (Ross & Williams, 2010) as a result of slow but continuous appositional growth (Atkinson, 2013). Indeed, computerized tomography (CT) scans have allowed the 3-D geometric analysis of landmarks of anatomic regions of the skull, and this has highlighted significant changes in the morphology of the aging human skull (Urban et al., 2016). Importantly, too, this research indicates that such changes are quite different by sex. Additionally, CT scans have also shown that the angle of the lower jaw drops as we age (Shaw et al., 2011). This age-related development occurs, however, earlier in women than in men. Thus, ratios involving lower face height should be affected with lower face lengthening in women in middle age (41–64) reducing sexual dimorphism, but the later lower face lengthening in men in older age (64+) potentially reinstating that dimorphism. Therefore, morphological change in skeletal structure and facial adiposity as a result of normal aging and hormonal changes including menopause may at least partially explain age-related changes in facial sexual dimorphism.
The study also supports the adoption of extended and more differentiated investigations into the phenotypic facial markers in nonhumans used in social interactions, primarily perhaps in terms of signals of dominance and submission. Certainly, previous research has evidenced the ability of untrained human participants to use static, nonexpressive facial cues to recognize accurately elements of chimpanzee personality related to extraversion (Kramer, King, & Ward, 2011). Similarly, human participants were able to recognize health information as well as information regarding extroversion, agreeableness, and emotional stability in chimpanzees, again from static, nonexpressive facial cues (Kramer & Ward, 2012). However, as far as we are aware, there have been just two comparative studies, involving brown capuchin monkeys and macaques, which have specifically utilized faciometrics (Borgi & Majolo, 2016; Lefèvre et al., 2014). Both have offered promising insights into the use of faciometrics in comparative studies from both an inter- and intraspecies perspective. The current study supports the proposition that further research could consider employing faciometrics beyond the fWHR while also recognizing the potentially diverging developmental trajectories of the faciometrics employed.
In summary, then, this research suggests that there is ample scope for further research using faciometrics in both human and nonhuman animals. We conclude, however, that despite the wealth of research in human facial sexual dimorphism, there has been relatively little research into the stability of such indices over time. We show that far from static, these indices change dynamically from early adulthood to late middle age. Additionally, the noted decline in facial dimorphism between the sexes is not consistent across all measures of it. Rather, it is evident that differing measures of sexual dimorphism follow distinct developmental trajectories. In view of the increasing literature into facial structure and associations with behaviors and the perceptions of others, it is important, therefore, that conclusions drawn from student-aged human samples are not generalized to older populations.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- Atkinson M. E. (2013). Anatomy of dental students (4th ed.). Oxford, England: Oxford University Press. [Google Scholar]
- Bird B. M., Valeska S., Cid Jofré V. S., Geniole S. N., Welker K. M., Zilioli S., Carré J. M. (2016). Does the facial width-to-height ratio map onto variability in men’s testosterone concentrations? Evolution and Human Behavior, 37, 392–398. [Google Scholar]
- Carré J. M. (2016). Evolution and Human Behavior, 37, 392–398. [Google Scholar]
- Carré J. M., McCormick C. M. (2008). In your face: Facial metrics predict aggressive behavior in the laboratory and in varsity and professional hockey players. Proceedings of the Royal Society B, 275, 2651–2656. doi:10.1098/rspb.2008.0873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carré J. M., McCormick C. M., Mondloch C. J. (2009). Facial structure is a reliable cue of aggressive behavior. Psychological Science, 20, 1194–1198. [DOI] [PubMed] [Google Scholar]
- Carré J. M., Morrissey M. D., Mondloch C. J., McCormick C. M. (2010). Estimating aggression from emotionally neutral faces: Which facial cues are diagnostic? Perception, 39, 356–377. [DOI] [PubMed] [Google Scholar]
- Cohen J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Lawrence Erlbaum. [Google Scholar]
- Danel D., Pawlowski B. (2007). Eye-mouth-eye angle as a good indicator of face masculinization, asymmetry, and attractiveness (Homo sapiens). Journal of Comparative Psychology, 121, 221–225. doi:10.1037/0735-7036.121.2.221 [DOI] [PubMed] [Google Scholar]
- Field A. P. (2009). Discovering statistics using SPSS (and sex and drugs and rock ‘n’ roll) (3rd ed.). London, England: Sage. [Google Scholar]
- Frackiewicz W. (2001). The aesthetics of the eyes and mouth position in a three-point face schema. Anthropological Review, 64, 93–100. [Google Scholar]
- Geniole S. N., Molnar D. S., Carré J. M., McCormick C. M. (2014). The facial width-to-height ratio shares stronger links with judgments of aggression than with judgments of trustworthiness. Journal of Experimental Psychology: Human Perception and Performance, 40, 1526–1541. [DOI] [PubMed] [Google Scholar]
- Grammer K., Thornhill R. (1994). Human (Homo sapiens) facial attractiveness and sexual selection: The role of symmetry and averageness. Journal of Comparative Psychology, 108, 233–242. [DOI] [PubMed] [Google Scholar]
- Greil H. (2006). Patterns of sexual dimorphism from birth to senescence. Collegium Antropologicum, 30, 637–641. [PubMed] [Google Scholar]
- Guégan J. F., Teriokhin A. T., Thomas F. (2000). Human fertility variation, size-related obstetrical performance and the evolution of sexual stature dimorphism. Proceedings of the Royal Society B: Biological Sciences, 267, 2529–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakstian A. R., Roed J. C., Lind J. C. (1979). Two-sample T2 procedure and the assumption of homogenous covariance matrices. Psychological Bulletin, 86, 1255–1263. [Google Scholar]
- Haselhuhn M. P., Ormiston M. E., Wong E. M. (2015). Men’s facial width-to-height ratio predicts aggression: A meta-analysis. PLoS ONE, 10, e0122637. doi:10.1371/journal.pone.0122637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselhuhn M. P., Wong E. M. (2011). Bad to the bone: Facial structure predicts unethical behavior. Proceedings of the Royal Society B. doi:10.1098/rspb.2011.1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehman E., Flake J. K., Freeman J. B. (2015). Static and dynamic facial cues differentially affect the consistency of social interactions. Personality and Social Psychology Bulletin, 1–12. doi:10.1177/0146167215591495 [DOI] [PubMed] [Google Scholar]
- Hehman E., Leitner J. B., Deegan M. P., Gaertner S. L. (2013).Facial structure is indicative of explicit support for prejudicial beliefs. Psychological Science, 24, 289–296. [DOI] [PubMed] [Google Scholar]
- Hehman E., Leitner J. B., Freeman J. B. (2014). The face–time continuum lifespan changes in facial width-to-height ratio impact aging-associated perceptions. Personality and Social Psychology Bulletin. doi:0146167214552791 [DOI] [PubMed] [Google Scholar]
- Hodges-Simeon C. R., Hanson Sobraske K. N., Samore T., Gurven M., Gaulin S. J. C. (2016). Facial width-to-height ratio (fWHR) is not associated with adolescent testosterone levels. PloS One. doi:10.1371/journal.pone.0153083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S., Bremme R. (2011). The effects of facial symmetry and sexually-dimorphic facial proportions in assessments of sexual orientation. Journal of Social, Evolutionary and Cultural Psychology, 5, 214–230. [Google Scholar]
- Jaffe C. A., Ocampo-Lim B., Guo W., Krueger K., Sugahara I., DeMott-Friberg R., Barkan A. L. (1998). Regulatory mechanisms of growth hormone secretion are sexually dimorphic. Journal of Clinical Investigation, 102, 153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R. S. S. (2015). Facial width-to-height ratio in a large sample of commonwealth games athletes. Evolutionary Psychology, 13, 197–209. [PubMed] [Google Scholar]
- Kramer R. S. S. (2017). Sexual dimorphism of facial width-to-height ratio in human skulls and faces: A meta-analytical approach. Evolution and Human Behavior, 38, 414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R. S. S., Jones A. L., Ward R. (2012). A lack of sexual dimorphism in width-to-height ratio in White European faces using 2D photographs, 3D scans, and anthropometry. PLoS ONE, 7, e42705. doi:10.1371/journal.pone.0042705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R. S. S., King J., Ward R. (2011). Identifying personality from the static, nonexpressive face in humans and chimpanzees: Evidence of a shared system for signaling personality. Evolution and Human Behavior, 32, 179–185. [Google Scholar]
- Kramer R. S. S., Ward R. (2012). Cues to personality and health in the facial appearance of chimpanzees (Pan troglodytes). Evolutionary Psychology, 10, 320–337. [PubMed] [Google Scholar]
- Law J., Bloor I., Budge H., Symonds M. E. (2014). The influence of sex steroids on adipose tissue growth and function. Hormone molecular biology and clinical investigation, 19, 13–24. doi:10.1515/hmbci-2014-0015 [DOI] [PubMed] [Google Scholar]
- Lefèvre C. E., Lewis G. J., Bates T. C., Dzhelyova M., Coetzee V., Deary I. J., Perrett D. I. (2012). No evidence for sexual dimorphism of facial width-to-height ratio in four large adult samples. Evolution and Human Behavior, 33, 623–627. [Google Scholar]
- Lefèvre C. E., Lewis G. J. (2013). Perceiving Aggression from Facial Structure: Further Evidence for a Positive Association with Facial Width-to-Height Ratio and Masculinity, but not for Moderation by Self-Reported Dominance. European Journal of Personality, 28, 530–537. [Google Scholar]
- Lefèvre C. E., Lewis G. J., Perrett D. I., Penke L. (2013). Telling facial metrics: Facial width is associated with testosterone levels in men. Evolution and Human Behavior, 34, 273–279. [Google Scholar]
- Lefèvre C. E., Wilson V. A. D., Morton F. B., Brosnan S. F., Paukner A., Bates T. C. (2014). Facial width-to-height ratio relates to alpha status and assertive personality in capuchin monkeys. PLoS ONE, 9, e93369. doi:10.1371/journal.pone.0093369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis G. J., Lefèvre C. E., Bates T. C. (2012). Facial width-to-height ratio predicts achievement drive in US presidents. Personality and Individual Differences, 52, 855–857. [Google Scholar]
- Little A. C., Jones B. C., Waitt C., Tiddeman B. P., Feinberg D. R., Perrett D. I. (2008). Symmetry is related to sexual dimorphism in faces: data across culture and species. PLoS One, 3. doi:org/10.1371/journal.pone.0002106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozener B. (2012). Facial width-to-height ratio in a Turkish population is not sexually dimorphic and is unrelated to aggressive behavior. Evolution and Human Behavior, 33, 169–173. [Google Scholar]
- Penton-Voak I. S., Jones B. C., Little A. C., Baker S., Tiddeman B., Burt D. M., Perrett D. I. (2001). Symmetry, sexual dimorphism in facial proportions and male facial attractiveness. Proceedings of the Royal Society of London Series B-Biological Sciences, 268, 1617–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pound N., Penton-Voak I. S., Surridge A. K. (2009). Testosterone responses to competition in men are related to facial masculinity. Proceedings of the Royal Society B—Biological Sciences, 276, 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricanek K., Tesafaye T. (2006, April). MORPH: A longitudinal image database of normal adult age-progression. Paper presented at IEEE 7th International Conference on Automatic Face and Gesture Recognition, Southampton, UK. [Google Scholar]
- Ross A. H., Williams S. E. (2010). Craniofacial growth, maturation, and change: Teens to midadulthood. Journal of Craniofacial Surgery, 21, 458–461. doi:10.1097/SCS.0b013e3181cfea34 [DOI] [PubMed] [Google Scholar]
- Scheib J. E., Gangestad S. W., Thornhill R. (1999). Facial attractiveness, symmetry and cues to good genes. Proceedings of the Royal Society B, 266, 1913–1917. doi:10.1098/rspb.1999.0866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw R. B., Katzel E. B., Koltz P. F., Yaremchuk M. J., Girotto J. A., Kahn D. M., Langstein H. N. (2011). Aging of the facial skeleton: Aesthetic implications and rejuvenation strategies. Plastic and Reconstructive Surgery, 127, 374–383. [DOI] [PubMed] [Google Scholar]
- Stevenson S., Thornton J. (2007). Effect of estrogens on skin aging and the potential role of SERMs. Journal of Clinical Interventions in Aging, 2, 283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirrat M., Perrett D. I. (2010). Valid facial cues to cooperation and trust: Male facial width and trustworthiness. Psychological Science, 21, 349–354. [DOI] [PubMed] [Google Scholar]
- Stirrat M., Perrett D. I. (2012). Face structure predicts cooperation: Men with wider faces are more generous to their in-group when out-group competition is salient. Psychological Science, 23, 718–722. [DOI] [PubMed] [Google Scholar]
- Urban J. E., Weaver A. A., Lillie E. M., Maldjian J. A, Whitlow C. T., Stitzel J. D. (2016). Evaluation of morphological changes in the adult skull with age and sex. Journal of Anatomy, 229, 838–846. doi:10.1111/joa.12247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung E. H., Zhang C., Albert P. S., Mumford S. L., Ye A., Perkins N. J.…Schisterman E. F. (2013). Adiposity and sex hormones across the menstrual cycle: The biocycle study. International Journal of Obesity 37, 237–243.doi:10.1038/ijo.2012.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentova J.V., Kleisner K., Havlicek J., Neustupa J. (2014). Shape differences between the faces of homosexual and heterosexual men. Archives of Sexual Behavior, 43, 353–361. [DOI] [PubMed] [Google Scholar]
- Yasui T., Yonetsu M., Tanaka R., Tanaka Y., Fukushima S., Yamashita T.…Araki T. (2013). In vivo observation of age-related structural changes of dermal collagen in human facial skin using collagen-sensitive second harmonic generation microscope equipped with 1250-nm mode-locked Cr: Forsterite laser. Journal of Biomedical Optics, 18, 31108. doi:10.1117/1.JBO.18.3.031108 [DOI] [PubMed] [Google Scholar]
- Ziomkiewicz A., Ellison P., Lipson S. F., Thune I., Jasienska G. (2008). Body fat, energy balance, and estradiol levels: A study based on hormonal profiles from complete menstrual cycles. Human Reproduction, 23, 2555–2563. [DOI] [PubMed] [Google Scholar]


