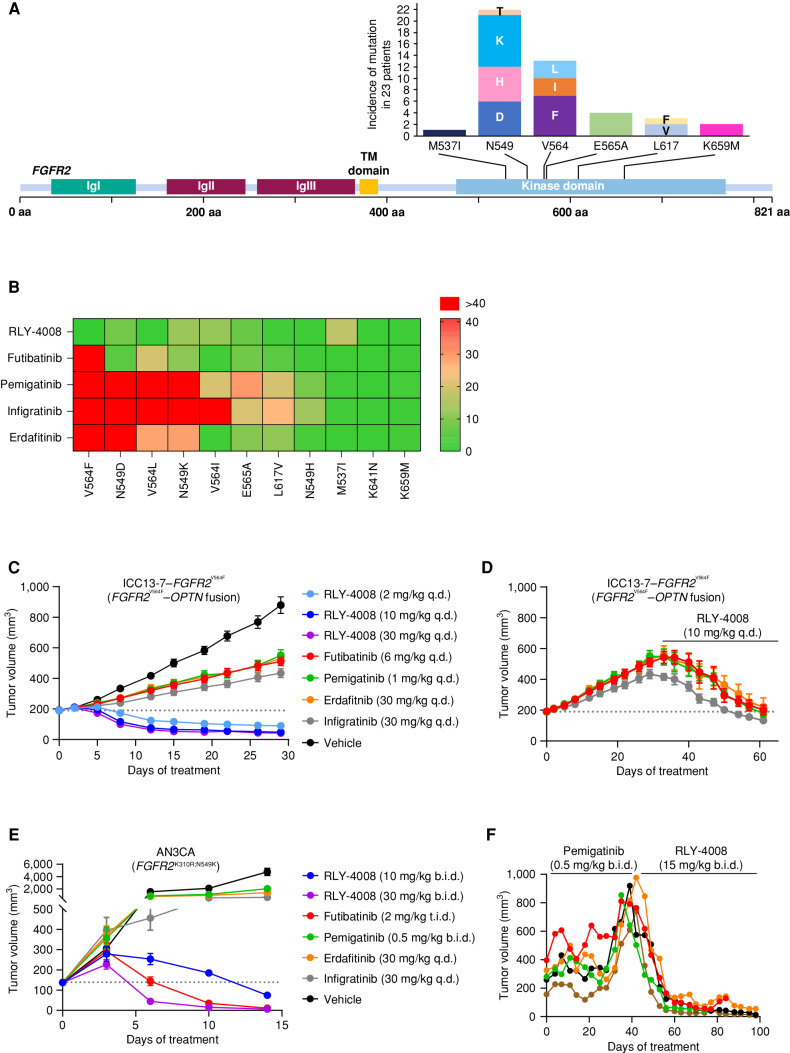
Figure 4.
RLY-4008 is active on mutations associated with acquired resistance to pan-FGFRi. A, Acquired resistance mutations in the FGFR2 kinase domain are commonly found in patients with FGFR2 fusion– or rearrangement–positive iCCA treated with pan-FGFRi. The graph indicates the number of times the indicated mutant allele was detected in tissue or ctDNA in 23 patients (out of 46) who developed FGFR2 kinase domain mutations at progression on pan-FGFRi. Figure art adapted from Varghese et al. and patient data are from Goyal et al. (23, 34). B, Heat map displaying the fold change in potency (IC50) for the indicated inhibitors against the indicated FGFR2 mutant as compared with FGFR2 WT. Numbering of mutant residues refers to the FGFR2 IIIc isoform to remain consistent with the usage of this nomenclature. Following 2 hours of incubation with the compound, FGFR2 inhibition was determined via pFGFR2 (Y653/654) HTRF assay, and IC50 values against FGFR2 WT and FGFR2 mutants were calculated. The average fold change of three independent experiments each containing two biological replicates was used to derive a heat map in GraphPad Prism. Fold change of one indicates equivalent potency on FGFR2 WT and the indicated FGFR2 mutant. C–E, Dotted line indicates tumor volume prior to initiation of treatment. C, Antitumor activity of RLY-4008 compared with futibatinib, pemigatinib, erdafitinib, and infigratinib in the ICC13-7–FGFR2V564F xenograft model (n = 8/group). Only RLY-4008 leads to tumor regression. Data are mean ± SEM. D, Following 28 days of treatment on the indicated inhibitors, animals on pan-FGFRi in the study shown in C were changed to treatment with RLY-4008 10 mg/kg once daily. Tumor regression was observed in all animals receiving RLY-4008. Data are mean ± SEM. E, Antitumor activity of RLY-4008 compared with futibatinib, pemigatinib, erdafitinib, and infigratinib in the AN3CA (FGFR2K310R;N549K) endometrial cancer xenograft model (n = 7/group). Only RLY-4008 and futibatinib treatment lead to tumor regression. Data are mean ± SEM. F, RLY-4008 overcomes acquired resistance to pemigatinib in vivo. Antitumor activity of pemigatinib followed by RLY-4008 in an FGFR2–TTC28 iCCA patient-derived xenograft model. Animals were dosed with pemigatinib for 40 days, followed by treatment with RLY-4008 from days 42–98. Each line represents one animal. b.i.d., twice daily; q.d., once daily; t.i.d., three times daily.