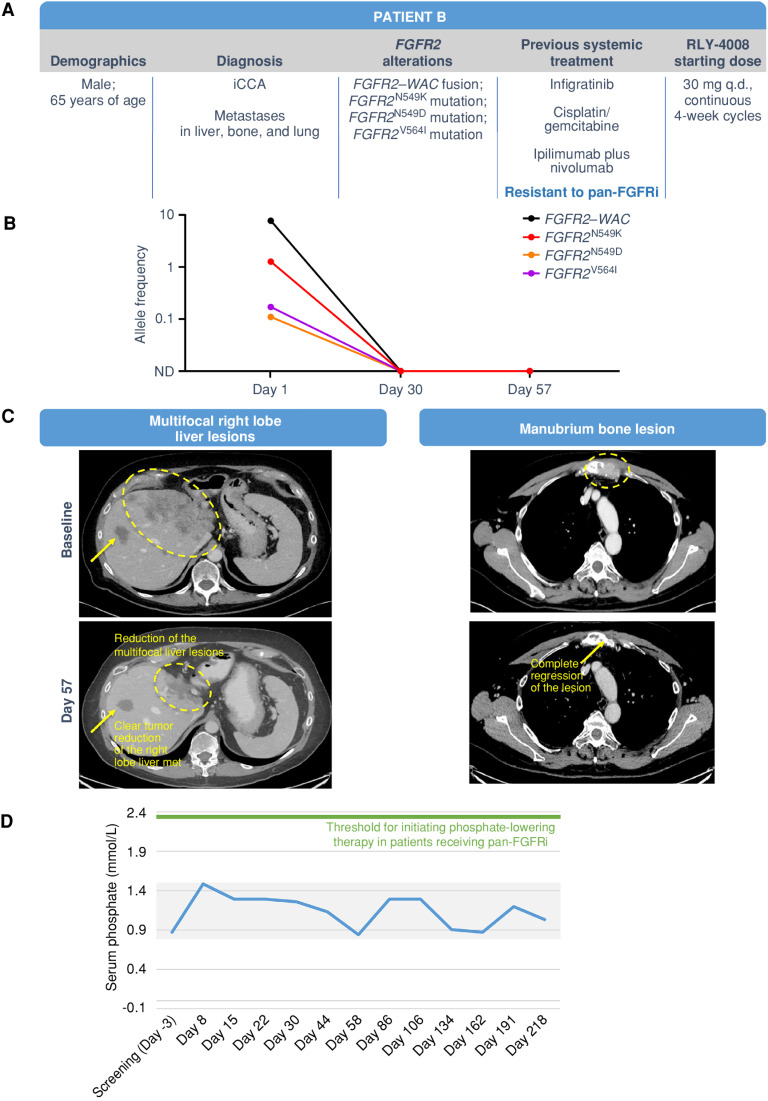
Figure 6.
Clinical response in a patient with pan-FGFRi–resistant iCCA with liver, bone, and lung metastases. The patient was treated with RLY-4008 starting at 30 mg once daily. A, Summary of key patient and disease characteristics. B, ctDNA analysis demonstrated complete clearance of FGFR2N549K, FGFR2N549D, and FGFR2V564 L clones by day 30. C, Left: CT scans of right lobe liver metastasis (arrow) and multifocal liver lesions (circled) at baseline (top) and on day 57 of RLY-4008 treatment (bottom) show a rapid, marked reduction in tumor volume. Right: CT scans of manubrium bone lesion at baseline (top) and on day 57 of RLY-4008 treatment (bottom) show complete regression of lesion. D, Serum phosphate during treatment with RLY-4008. Shaded area represents the normal range for serum phosphate (0.8–1.5 mmol/L). ND, not detectable; q.d., once daily.