FIGURE 2.
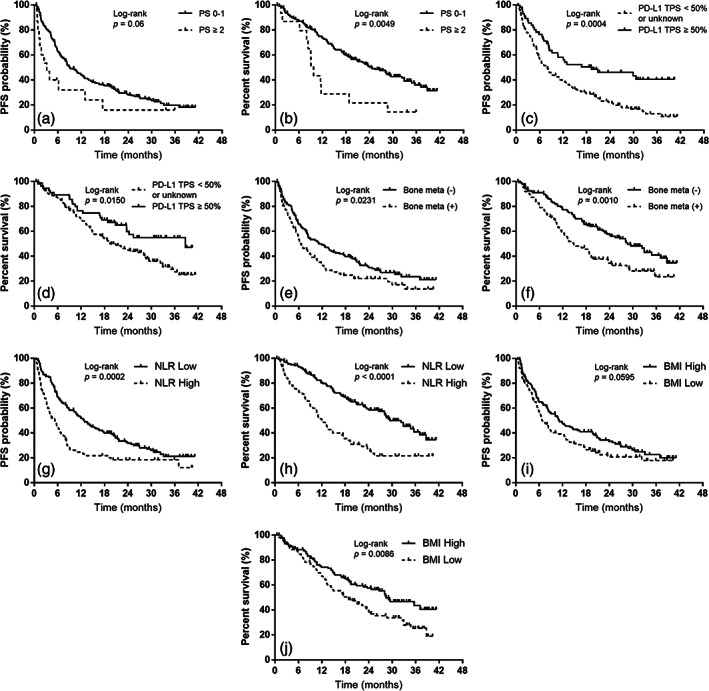
Kaplan–Meier curves for progression‐free survival (PFS) and overall survival (OS) according to performance status (PS) at the start of pembrolizumab plus platinum and pemetrexed treatment, programmed death ligand‐1 tumor proportion score (PD‐L1 TPS), presence of bone metastases at initial treatment, neutrophil‐to‐lymphocyte (NL) ratio, and body mass index (BMI). (a) PFS according to the PS at the start of pembrolizumab plus platinum and pemetrexed treatment (PS 0–1, median PFS: 9.0 months; PS ≥2, median PFS: 3.1 months). (b) OS according to the PS at the start of pembrolizumab plus platinum and pemetrexed treatment (PS 0–1, median OS: 24.3 months; PS ≥2, median OS: 9.7 months). (c) PFS according to PD‐L1 TPS (PD‐L1 TPS ≥50%, median PFS: 19.2 months; PD‐L1 TPS < 50% or unknown, median PFS: 7.7 months). (d) OS according to PD‐L1 TPS (PD‐L1 TPS ≥50%, median OS: 38.7 months; PD‐L1 TPS < 50% or unknown, median OS: 19.4 months). (e) PFS according to the presence of bone metastases at initial treatment (without bone metastases at initial treatment, median PFS: 11.2 months; with bone metastases at initial treatment, median PFS: 6.4 months). (f) OS according to the presence of bone metastases at initial treatment (without bone metastases at initial treatment, median OS: 28.6 months; with bone metastases at initial treatment, median OS: 14.8 months). (g) PFS according to the NL ratio (NL ratio <5, median PFS: 12.8 months; NL ratio ≥5, median PFS: 5.3 months). (h) OS according to the NL ratio (NL ratio <5, median OS: 29.4 months; NL ratio ≥5, median OS: 12.0 months). (i) PFS according to BMI (BMI ≥22.0, median PFS: 11.2 months; BMI < 22.0, median PFS: 6.8 months). (j) OS according to BMI (BMI ≥22.0, median OS: 28.4 months; BMI < 22.0, median OS: 18.4 months).
