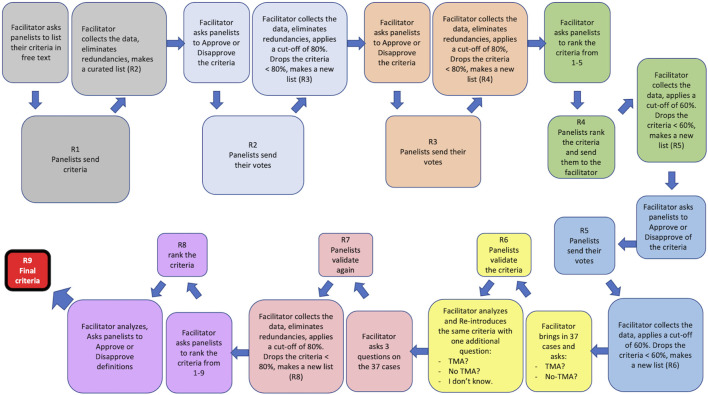
FIGURE 1.
The Delphi process applied to this study. Nine rounds of survey (R1–R9) were designed. At the beginning of each round or R, the facilitator presented the panelists with the results (criteria) obtained from the previous R and asked them to either approve/disapprove of the listed criteria or to rank them. The panelists individually responded to this call and sent their votes to the facilitator who would collect the responses, eliminate redundancies, and apply a cut-off (80% or 60%) to that R. The results of the cut-off application were then shared with the panelists. A new list composed of all criteria that were above the cut-off was made by the facilitator and presented in the next R to the panelists. R6 and R7 were two rounds during which the criteria obtained from R5 were validated against 37 real-life cases by the panelists. R9 was a control round during which the integrity of the entire Delphi process was assessed. R9 was used to fine tune the definitions of the lesions that the panelists had difficulty with, during the validation R and was therefore called the Definition R.