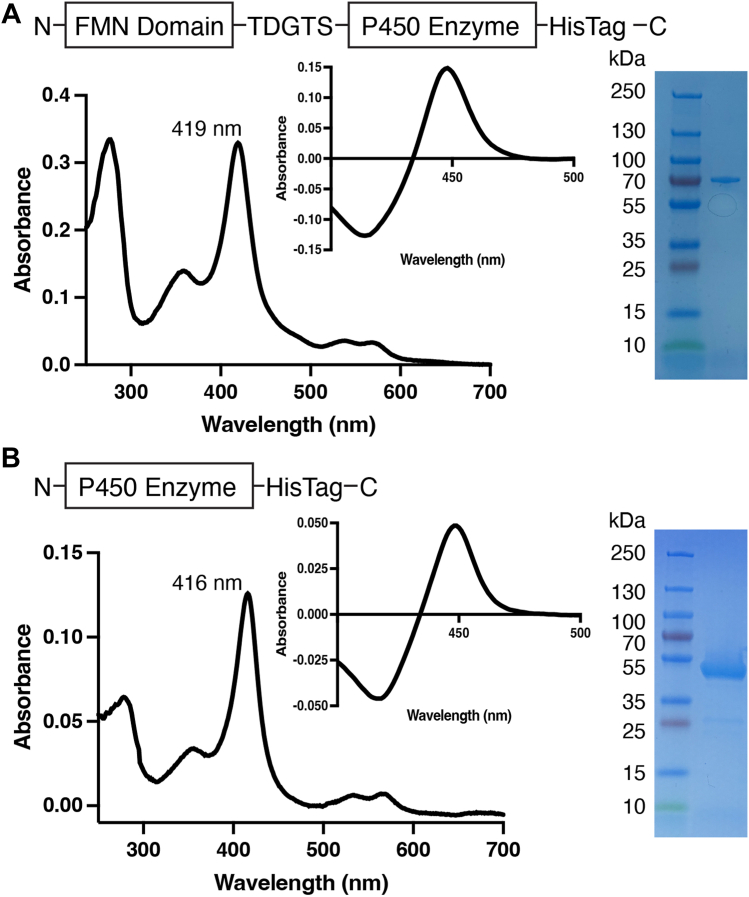
Figure 1.
Generation and characterization ofrepresentative FMND/P450 fusion enzyme compared withthe corresponding isolated P450 enzyme.A, fusion enzymes consisted of the reductase FMN domain plus a five-amino-acid linker plus the catalytic P450 domain with a histidine tag (top). This resulted in purified protein with a water-bound Soret peak in the absolute spectrum (main image) and a typical reduced-carbon monoxide difference spectrum (inset). The purified protein runs on an SDS-PAGE gel at the expected molecular weight of 75 to 77 kDa (right). B, the individual P450 enzymes that the fusions were compared with consisted of only the catalytic P450 domain with a histidine tag (top). This resulted in purified protein with a water-bound Soret peak in the absolute spectrum (main image) and a typical reduced-carbon monoxide difference spectrum (inset). The purified protein runs on an SDS-PAGE gel at the expected molecular weight of 54 to 56 kDa (right). The specific examples shown are for (A) FMND/CYP2A6 and (B) CYP2A6. Fig. S3 shows these data for all proteins, including repetition of FMND/CYP2A6 and CYP2A6 to facilitate comparisons.