Table 2. Composition of the Emulsion Formulationsa.
| Formulation | Dispersed Phase (% v/v) | Stabilizer (% w/w) | DLM (% w/w) | DLM loading (%) |
|---|---|---|---|---|
| F1 | 15 | 1.5 | 1.0 | 40 |
| F2 | 15 | 1.0 | 0.67 | 40 |
| F3 | 15 | 0.5 | 0.33 | 40 |
| F4 | 20 | 2.0 | 1.33 | 40 |
| F5 | 25 | 2.5 | 1.67 | 40 |
| F6 | 30 | 3.0 | 2.0 | 40 |
| F7 | 40 | 4.0 | 2.67 | 40 |
The dispersed phase was dichloromethane
and the continuous phase was aqueous. The stabilizer was either a
cellulosic polymer or a 1:1 mass ratio of a cellulosic polymer to
lecithin as a co-stabilizer. The dispersed phase content of each formulation
is reported as a volume fraction, 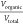 , where Vorganic is the volume of dichloromethane and Vtotal is the total volume of the organic and aqueous phases
of the emulsion.
Stabilizer and DLM concentrations are reported as a weight percent
of the total mass of the liquid emulsion formulation. Drug loading
is reported as a weight percent of the total mass of solid (nonvolatile)
components of the emulsion,
, where Vorganic is the volume of dichloromethane and Vtotal is the total volume of the organic and aqueous phases
of the emulsion.
Stabilizer and DLM concentrations are reported as a weight percent
of the total mass of the liquid emulsion formulation. Drug loading
is reported as a weight percent of the total mass of solid (nonvolatile)
components of the emulsion, 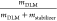 , where m denotes the mass
of the respective components. For formulations containing two stabilizers
(i.e., lecithin and a cellulosic stabilizer), the mass ratio of the
stabilizers was 1:1.
, where m denotes the mass
of the respective components. For formulations containing two stabilizers
(i.e., lecithin and a cellulosic stabilizer), the mass ratio of the
stabilizers was 1:1.
