Abstract
Neurological conditions such as Alzheimer’s disease (AD) and related dementias (ADRD) present with many challenges due to the heterogeneity of the related disease(s), making it difficult to develop effective treatments. Additionally, the progression of ADRD-related pathologies presents differently between men and women. With two-thirds of the population affected with ADRD being women, ADRD has presented itself with a bias toward the female population. However, studies of ADRD generally do not incorporate sex-based differences in investigating the development and progression of the disease, which is detrimental to understanding and treating dementia. Additionally, recent implications for the adaptive immune system in the development of ADRD bring in new factors to be considered as part of the disease, including sex-based differences in immune response(s) during ADRD development. Here, we review the sex-based differences of pathological hallmarks of ADRD presentation and progression, sex-based differences in the adaptive immune system and how it changes with ADRD, and the importance of precision medicine in the development of a more targeted and personalized treatment for this devastating and prevalent neurodegenerative condition.
Keywords: B lymphcoytes, T lymphocytes, Dementia, Estrogen, Tesosterone
1. Introduction
Life expectancy in the United States from 1900 to 2020 has increased from 47.3 to 77.0 years of age (Fisk, 2003; CDC, 2022). While improvements in biomedical research and medicine have led to this increase in life expectancy, it has also brought about new health crises among the elderly. One prevalent example is increasing rates of dementia, which is a syndrome that impairs cognitive abilities leading to a reduction in independent living. Alzheimer’s disease (AD), a neurodegenerative disease, is the most common form of dementia and accounts for approximately 60–80% of all dementia cases (Alzheimers Dement., 2022). The prevalence of AD increases with age and affects about 10–30% of the population over the age of 65 years old (Masters et al., 2015). The pathological hallmarks of AD are amyloid β (Aβ) deposition in the brain, neurofibrillary tangles due to accumulation of hyper-phosphorylated and misfolded τau protein, and neuronal loss. There are many risk factors associated with Alzheimer’s disease and related dementias (ADRD) including age, sex, cardiovascular diseases (CVD), cerebrovascular diseases, and metabolic diseases (R, 2019).
Recent developments suggest that systemic inflammation affects the central nervous system (CNS) to cause neurodegeneration and cognitive decline. Pro-inflammatory cytokines such as interleukin (IL)-6, tumor necrosis factor (TNF), C-reactive protein (CRP), as well as cytokine receptors, are elevated in subjects with mild cognitive impairment (MCI; i. e., prodromal for AD) and ADRD (Walker et al., 2019). The interest in systemic inflammation has mostly focused on innate immune cells, while investigation of the involvement of the adaptive immune system remains understudied. Furthermore, immune responses are impacted by sex, but how that may affect inflammation and ADRD interactions is also not often considered. Sex differences have been seen in the incidence and pathophysiology of ADRD, as the incidence of ADRD are disproportionate with the highest incidence being in women (Alzheimers Dement., 2022). Additionally, several risk factors for ADRD such as CVDs, cerebrovascular and metabolic diseases differ between women and men (Dong et al., 2022; Yoshida et al., 2022).
In this review, we summarize the current knowledge of sex differences in systemic inflammation due to adaptive immunity in ADRDs. We discuss the differences between men and women in their ADRD pathology and how they are affected by sex hormones. We specifically discuss how sex differences affect the adaptive immune system and the potential impact in the development of ADRD. Finally, we discuss the importance of sex differences and inflammation due to adaptive immune system changes in the development of precision medicine in ADRD. As most studies have not considered sex as a nonmodifiable risk factor of ADRD, we think it is critical in understanding not only the pre-clinical development of the disease but potentially also in targeting both clinical trials and patient populations for therapeutics.
2. Overview of sex differences in the development of Alzheimer’s disease and related dementias (ADRD)
Nearly two-thirds of Americans living with ADRD are women, and there is increasing evidence that supports sex-based differences in the risk of ADRD development, its presentation, and progression. Genetic factors such as apolipoprotein ε (APOε) 4 gene, a strong risk factor in the development of ADRD, show a greater association with women than men (Fig. 1) (Altmann et al., 2014). Women at early stages of AD dementia or MCI exhibit lower Mini-Mental State Examination (MMSE) scores and show a faster rate of cognitive decline from MCI to AD than men (Gamberger et al., 2017; Tifratene et al., 2015; Pradier et al., 2014). Women between the age of 55 and 70 years, as well as carriers of the APOε4 allele, are at greater risk of MCI than men, though the protective effects of APOε2/ε3 allele in the development of AD are also greater in women than in men (Neu et al., 2017). Other studies showed that a combination of amyloid β (Aβ) burden and APOε4 allele expression resulted in a faster decline of cognition in women compared to men (Buckley et al., 2018; Holland et al., 2013) and an even greater decline in women over a period of 8 years (Lin et al., 2015). A meta-analysis genetic study identified one intergenic single-nucleotide polymorphism (SNP) on chromosome 7 with a strong association of neurofibrillary tangles only amongst men but not women (Dumitrescu et al., 2019a). These findings support the sex-based differences in genetic factors as prodromal for cognitive impairment and diagnosis of ADRD. Recently, a genome-wide association study (GWAS) identified 43 new genes associated with AD diagnosis, although sex differences were not considered (Bellenguez et al., 2022). While APOε4 appears to be a lead therapeutic target in the prevention or progression of dementia, more clinical research is required to determine the impact of sex and/or age.
Fig. 1.
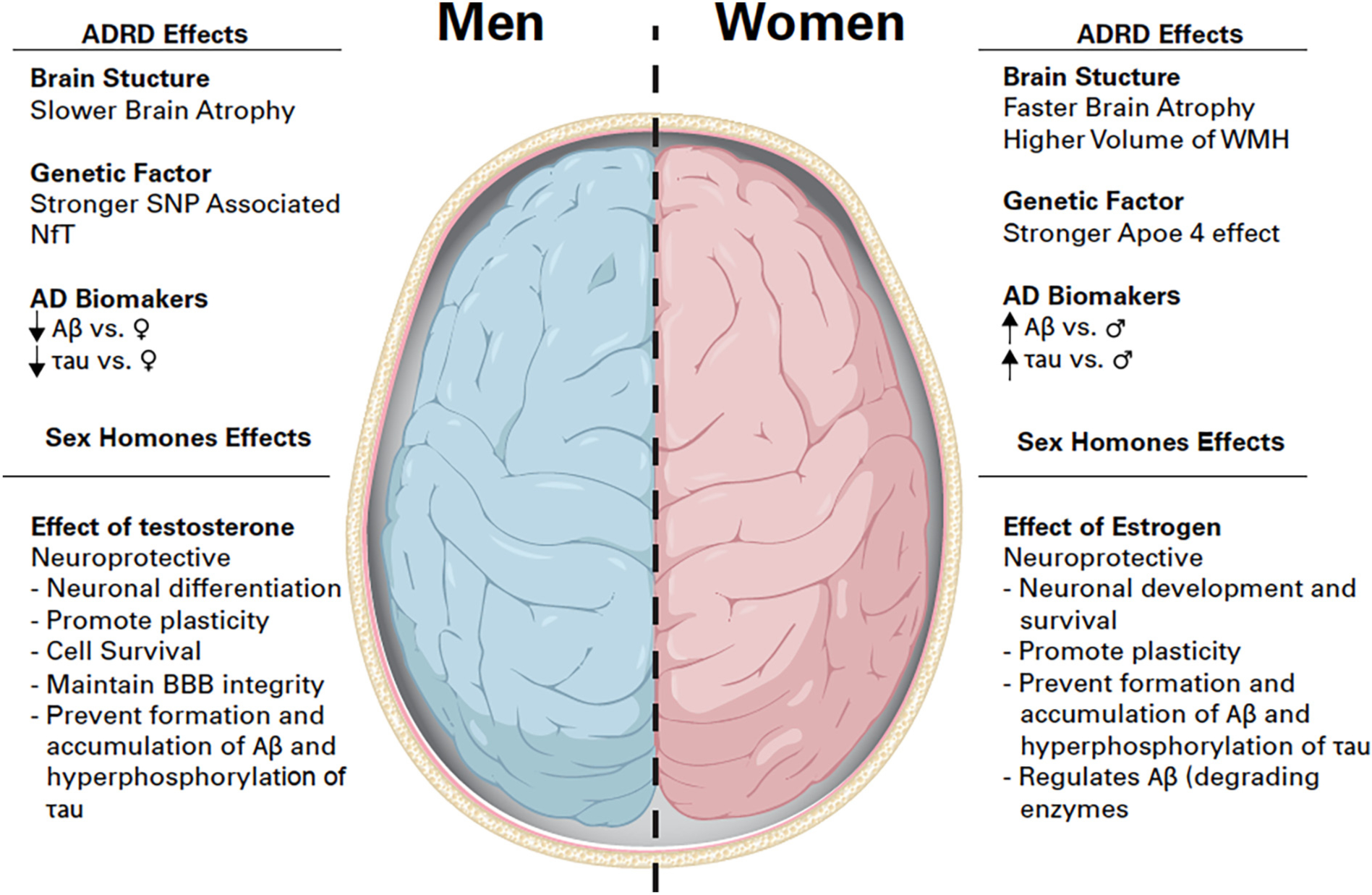
Summary of the effects of Alzheimer’s disease and related dementia and sex hormones on the brain. ADRD effects on Brain structure, genetic factors and Alzheimer’s disease biomarkers, and Sex hormones effects on the brain in men and women. Symbols: ↑increase, ↓decrease,♀women, ♂ men. Abbreviations: ADRD: Alzheimer’s disease and related dementia, SNP: single nucleotide polymorphisms, WMH: White matter hyperintensity, Aβ: Amyloid Beta, BBB: Blood-brain barrier.
Known risk factors for ADRD include cardiovascular and cerebrovascular events such as hypertension, stroke, and microinfarcts. These risk factors are generally more prevalent in women after menopause and over the age of 60 (Longstreth Jr et al., 2009; Choi et al., 2017). In a recent systematic review investigating sex differences in blood pressure and ADRD, Blanken et al. identified six different studies where women with a diagnosis of hypertension were at higher risk of ADRD than men (Blanken and Nation, 2020). Cerebral small vessel diseases can manifest as hypertensive vascular lesions that can be detected by Magnetic Resonance Imaging (MRI) as white matter hyperintensities (WMH), or localized areas of edema, inflammation, and potential demyelination (Prins and Scheltens, 2015). Many have reported a higher total volume of WMH in women compared to men (de Leeuw et al., 2001; van den Heuvel et al., 2004; Sachdev et al., 2009; Fatemi et al., 2018) but regardless of sex, WMH increases with age and are associated with cognitive decline and dementia (Benedictus et al., 2015; Zhuang et al., 2018). In addition, brain atrophy identified through MRI is a widely accepted marker of ADRD and also demonstrates a sex-based difference in the rate and region of atrophy. The brain structure of patients with MCI show greater and faster rates of atrophy in women compared to men in a one-year period (Hua et al., 2010), and studies looking at hippocampal volume from participants with ADRD confirmed a faster decline in hippocampal volume in women (Burke et al., 2019; Koran et al., 2017). Holland et al. identified that the amygdala, entorhinal, inferior parietal, middle temporal cortices, and the whole brain in general atrophied much faster in women with MCI and AD than in men (Holland et al., 2013). Although limited, this research shows that ADRD risk factors have sex-based effects, and that primary neuropathological outcomes, including brain size, structure, and presence of WMH are much more affected in both the early and late stages of dementia in women than in men, which suggest a wider and greater impact in ADRD in women than in men.
Investigation of canonical mechanisms of ADRD development showed that sex-based differences in Aβ burden and in the burden of neurofibrillary tangles or τau hyperphosphorylation are inconclusive. Earlier work from postmortem brain on sex differences in Aβ and τau found sex differences in Aβ and τau accumulation to be higher in women than in men, especially between the age of 60 and 75 (Corder et al., 2004; Salehi et al., 1998). More recent studies investigating Aβ in MCI and AD found no clear sex differences in Aβ burden. Specifically, Aβ levels in the cerebrospinal fluid (CSF) or Aβ levels identified by positron emission tomography (PET) in patients with either MCI or AD did not reflect any association with sex (Jansen et al., 2015; Mattsson et al., 2017; Babapour Mofrad et al., 2020). Early work on τau found higher numbers of neurofibrillary tangles in women compared to men in certain cortical areas of the brain in postmortem tissues (Barnes et al., 2005). Other work supports a higher level of neurofibrillary tangles in women than in men (Holland et al., 2013; Corder et al., 2004), though a few studies showed no sex differences in τau protein (Mattsson et al., 2017). CSF and plasma levels of τau and phospho-τau (pTau) are also higher in women than men with AD, including in association with APOε4 (Babapour Mofrad et al., 2020; Hohman et al., 2018). A more recent study confirmed higher CSF and plasma levels of τau phosphorylated at threonine 181 (pTau181), a biomarker that shows a better association with Aβ accumulation in the brain, in women were associated with a higher incidence of dementia (Tsiknia et al., 2022). While these studies have very large participants in their cohorts, the sex-based differences in Aβ and τau are still limited and mostly inconclusive, though different animal and in vitro studies demonstrate regulation of both Aβ and τau by testosterone and estrogen, which we discuss below.
3. The role of sex in the pathogenesis of ADRD
3.1. The role of sex hormones in the pathogenesis of ADRD
3.1.a. Androgens
Testosterone and dihydrotestosterone (DHT) are the main androgens in mature males. Testosterone is synthesized from cholesterol to be either aromatized to estradiol or converted to DHT (Andersson et al., 1989; Marchetti and Barth, 2013). Though aging is a defining risk factor for ADRD, it is also associated with the loss of androgens in men. A decrease in androgens in men over the age of 65 leads to a decrease in muscle mass, bone loss, change in mood, and cognitive function (Baumgartner et al., 1999; Mohamad et al., 2016; Cherrier et al., 2009). Androgens have effects on brain morphology and beneficial effect on brain cells (Fig. 1). They stimulate neuronal differentiation, maintain neuronal morphology, and promote synaptic density. In the hippocampus of the brain, a region associated with learning and memory, testosterone and DHT improve new neuronal cell survival but not cell proliferation (Spritzer and Galea, 2007; Swift-Gallant et al., 2018). Androgens also mediate neuroprotective effects on neurons through redox homeostasis regulation by increasing mitochondrial production of ATP and antioxidant activity (Grimm et al., 2014; Ahlbom et al., 2001). Critically for aging, and especially associated with cerebral small vessel diseases and WMH, androgens maintain blood-brain barrier (BBB) integrity (Atallah et al., 2017). Thus, with age, the beneficial effects of androgens in the brain decrease; therefore, increasing susceptibility to cognitive decline.
As cognitive decline has long been linked with androgen deficiency in older men, early studies reported that low levels of testosterone associated with decline in cognitive functions using neuropsychiatric tests including the MMSE, Trails B, and selective reminding test (Moffat et al., 2002; Hogervorst et al., 2010). In a longitudinal study over a period of 5 years, Hsu et al. confirmed that a decline in androgen levels over time was predictive of a decline in cognitive function (Hsu et al., 2015), though again other studies did not show a correlation between low androgen levels in serum or plasma to cognitive decline (LeBlanc et al., 2010). However, Ford et al. demonstrated that only men with very low levels of testosterone were at high risk of increased cognitive decline compared to those with a slightly lower level of testosterone (Ford et al., 2018). This inconsistency could be attributed to the lack of a pre-defined range of androgen levels that correlate with cognitive decline. Thus, more longitudinal studies are needed to identify the pathological levels of androgens that predict cognitive decline.
Androgen specifically mediates neuroprotective effects by preventing the formation and accumulation of the two primary lesions associated with AD: accumulation of Aβ and hyperphosphorylation of τau protein. Several mechanisms of Aβ clearance induced by androgen through the androgen receptor (AR) have also been identified. In mice, androgen suppressed IL-1β and TNF expression in microglia, increasing Aβ degradation by microglia phagocytosis and clearance (Yao et al., 2017). Androgen also increased the Aβ-catabolizing enzyme neprilysin to accelerate Aβ clearance, and heat shock protein (HSP)70 to inhibit Aβ accumulation (Yao et al., 2008; Lau et al., 2014; Zhang et al., 2004). Androgens prevented τau hyperphosphorylation by increasing phosphorylation to protein kinase B (Akt), a serine/threonine-specific protein kinase, and glycogen synthase kinase 3 beta (GSK3β) (Rosario et al., 2010; Yao et al., 2022). Testosterone can also clear Aβ and prevent τau hyperphosphorylation through conversion to estrogen by the converting enzyme aromatase and/or acting through the estrogen receptor (ER) (Cui et al., 2013). Although the protective effects of androgen in the brain are clear, the extent of these effects still remains to be elucidated. This includes whether targeting androgens in males would be an appropriate therapeutic candidate.
3.1.b. Estrogens
There are three different forms of estrogens in females: estrone (E1), estradiol (E2 or 17β-estradiol), and estriol (E3) (Cui et al., 2013). E1 is characterized as a weak estrogen and is produced in adipose tissues after menopause. E2 plays an important role during the pre-menopausal phase and is mainly produced by the ovaries. It is the most potent and most physiologically-relevant estrogen (Cui et al., 2013). E3 is produced by the placenta and plays an important role during pregnancy. For the purpose of this review, the term estrogen will refer to estradiol (E2).
The actions of estrogen are mediated through the estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ). Estrogen has beneficial effects on different tissues in the body, including an important role in bone formation and maturation in both men and women (Vaananen and Harkonen, 1996). Estrogen also displays protective effects in the brain as it protects against neuronal damage, regulates neuronal development and survival, and promotes neuronal plasticity (Kretz et al., 2004; Azcoitia et al., 2001; Martinez-Cerdeno et al., 2006) (Fig. 1). Estrogen regulates spinogenesis in the hippocampus (Woolley et al., 1990). The neuroprotective effects of estrogens from ischemic stroke, a main risk factor for ADRD, are ERα-dependent (Dubal et al., 2001). Estrogen mediates sex-specific pathology, as a decline in estrogen levels after menopause can result in cognitive decline and the development of ADRD in women. As previously stated, testosterone is aromatized to estrogen, and women express a low level of testosterone compared to men and testosterone decreases with age (Zumoff et al., 1995; Davis and Wahlin-Jacobsen, 2015). This raises the question of insufficient amount of testosterone to convert to estrogen and could also contribute to cognitive decline in women. Several studies support the hypothesis that reduced estrogen levels increase the risk for ADRD.
Mechanistically, estrogen regulates Aβ and τau phosphorylation. In human neuroblastoma cell lines, estrogen induces τau dephosphorylation in proline and non-proline sites (Alvarez-de-la-Rosa et al., 2005). Zhang et al. also showed that estrogen prevents τau hyperphosphorylation in a dose-dependent manner (Zhang and Simpkins, 2010). On the other hand, in a mouse model of AD, estrogen treatment in ovariectomized 3xTg-AD mice did not significantly reduce τau hyperphosphorylation but prevented accumulation of Aβ, a potential additional mechanism of protection (Carroll et al., 2007). Genetics expression profiling of the hippocampus in peri-menopausal animal models identified decreased expression of genes associated with Aβ degradation under low levels of estrogen (Yin et al., 2015). These findings are supported by other studies wherein estrogen regulates Aβ-degrading enzymes (Merlo and Sortino, 2012; Xiao et al., 2009; Nord et al., 2010). Ultimately, the beneficial effects of estrogen on neurons, as well as the ability for Aβ degradation and clearance, has made estrogen a target for a therapeutic approach to prevent cognitive decline and AD diagnosis at the early stages of menopause.
3.2. Hormone therapy
There is strong evidence supporting low sex hormones in women and men as a key risk factor for cognitive decline and AD. However, clinical studies on estrogen replacement therapy (ERT) and testosterone replacement therapy (TRT) have been contradictory and inconclusive. In some clinical studies, women over the age of 60 who received ERT exhibited a decreased risk of developing cognitive decline and AD, and the decrease in risk was even greater with prolonged ERT treatment and in some cases, increased dosage (Kawas et al., 1997; Tang et al., 1996; Paganini-Hill and Henderson, 1996). On the other hand, other clinical studies found no change attributed to ERT or worsening of the risk of dementia. This includes the Women’s Health Initiative Memory Study (WHIMS), where women who received ERT had a significantly increased risk of developing cognitive decline or dementia (Craig et al., 2005). The Cache County Study showed no beneficial effect of ERT on cognitive decline unless the treatment exceeded ten years (Zandi et al., 2002). These discrepancies have been mostly attributed to the time from the start of menopause to the start of ERT. In Henderson et al., the protective effect of ERT was age dependent, where women between the ages of 50–63 years undergoing ERT had a decreased risk of developing cognitive decline while older women did not have any beneficial effects (Henderson et al., 2005). Other studies confirmed findings from Henderson et al. and narrowed the age window to 5 years after menopause (Shao et al., 2012; Whitmer et al., 2011). Together, these findings support ERT as a therapeutic approach for women, however, efficacy may have a clear reliance on start with relation to menopause.
Studies also support testosterone supplements as a therapeutic approach due to its impact on neuroprotection (i.e. enhanced Aβ clearance), and association with ADRD diagnosis in men with lower testosterone. TRT in men improved cognitive function as measured by MMSE, depression scores, and overall cognition (Wahjoepramono et al., 2016; Cherrier et al., 2015; Jung and Shin, 2016). However, Resnick et al. investigated a larger daily dose of testosterone that did not improve cognitive function in men with low testosterone levels (Resnick et al., 2017). The contradictory finding could again be attributed to the difference in age as Resnick et al.’s study had men with an average age of 72.3 years while the average age in other studies were 67 or younger. In general, a recent meta-analysis supported the beneficial effect of TRT to prevent cognitive decline (Tan et al., 2019). Although sex hormone deficiency impacts ADRD, molecular mechanisms must be investigated more deeply so that hormone therapy can become an effective therapeutic approach to improve cognitive function.
3.3. The role of sex chromosomes in the pathogenesis of ADRD
3.3.a. X chromosome
The X chromosome is one of the two sex chromosomes in humans and is highly conserved in mammals as it is present in both males and females. The X chromosome accounts for 3–5% of the human genome and carries about 1200 genes. Since females carry two copies of the X chromosome, one copy of the X chromosome undergoes a silencing mechanism known as X chromosome inactivation (XCi) in order to regulate gene dosage. The frequency of the X chromosome loss increases with age. At ages less than 16 years old, the loss rate is at 0.07%, whereas at over 65 years of age, the rate of loss increases to 7.3% (Russell et al., 2007). There is increasing evidence to support the X chromosome in ADRD pathogenesis. Several studies have shown abnormal behavior of the X chromosome in subjects with AD. This includes an increase in premature centromere division (PCD) on the X chromosome in peripheral blood mononuclear cells (PBMCs) from AD subjects, with a higher percentage of PCD in women than in men (Spremo-Potparevic et al., 2004), a PCD frequency three times higher in AD subjects than in normal controls in their frontal cortical neurons (Spremo-Potparevic et al., 2008), and an X chromosome aneuploidy in the postmortem brain twice higher in AD patients (Yurov et al., 2014). Additionally, in a more recent study, 29 gene expressions on the X chromosomes were associated with cognitive changes only in women, not men, and 3 genes were associated with neuropathological τau burden only in men (Davis et al., 2021).
Several genes located on the X chromosomes have been shown to escape XCi and also play a role in ADRD. Protocadherin 11 X-linked (PCDH11X) is a gene that regulates cell-cell recognition and is highly expressed in the brain (Kahr et al., 2013). A GWAS study identified a SNP on Xq21.3 in PCDH11X that is associated with late-onset Alzheimer’s disease (LOAD) (Carrasquillo et al., 2009). However, two other GWAS studies found no association between PCDH11X and LOAD (Beecham et al., 2010; Miar et al., 2011). Nevertheless, these contradictory findings still leave PCDH11X as a candidate gene to be investigated in the development of ADRD. Ubiquitin specific peptidase 11 (USP11) inactivation has also been associated with AD. USP11 is a deubiquitinase involved in chromatin condensation, genomic stability, and cell survival (Liao et al., 2022). USP11 had been shown to escape inactivation and increase τau pathology in AD brains, mostly in women (Yan et al., 2022). Additionally, XCI of Lysine Demethylase 6A (KDM6A), which is involved in gene transcription (Yi et al., 2022), was associated with cognitive resilience in women with AD but not in men, suggesting a protective effect of KDM6A overexpression in ADRD development (Davis et al., 2020). With only three X-linked genes that have been directly studied in relation to ADRD development, the role of the X chromosome and associated genes in the development of ADRD remains understudied.
3.3.b. Y chromosomes
The Y chromosome contains the sex-determining region (SRY) gene responsible for triggering male development. The Y chromosome is 3 times smaller than the X chromosome and carries fewer genes (Quintana-Murci and Fellous, 2001; Charlesworth, 2003). Loss of Y chromosome (LOY) has been associated with aging in men (Forsberg et al., 2017). Additionally, LOY has been associated with age-related diseases including ADRD (Guo, 2021; Caceres et al., 2020). Transcriptomic data analysis found that extreme downregulation of chromosome Y in the brain was associated with age and AD (Caceres et al., 2020), and a study by Dumanski et al. demonstrated that LOY in blood cells was associated with AD in men (Dumanski et al., 2016). Further investigation identified Natural Killer (NK) Cells to be more affected by LOY in men with AD (Dumanski et al., 2021). Finally, a more recent study investigating LOY in the brain cells of subjects with neurodegenerative diseases found a significant LOY in microglia compared to other brain cells (Vermeulen et al., 2022). An additional study of induced pluripotent stem cells (iPSCs) of subjects with familial AD and carriers of the presenilin 1 (PSEN) E280A mutation showed that these subjects developed LOY and had increased Aβ42 levels (Mendivil-Perez et al., 2019). These studies provide evidence of the involvement of LOY in AD, however, more studies are needed to determine the extent of LOY in ADRD.
4. Sex-based differences in the adaptive immune system
Sex hormones also impact the immune system (Fig. 2), including the adaptive immune system which provides a second line of defense against recurring exposure to pathological microorganisms. Adaptive immunity is comprised of T and B lymphocytes and natural killer T (NKT) cells. The activation of these effector cells is tightly regulated and involves an interaction between antigen-presenting cells (APCs), generally of the innate immune system, and the T and B lymphocytes that facilitate pathogen-specific effector responses (Cano and Lopera, 2013). APCs are a heterogeneous group of immune cells that mediate the immune response by processing of antigens through phagocytosis or receptor-mediated endocytosis and presenting those antigens for recognition by T cells to initiate the adaptive immune response. These cells include macrophages, dendritic cells, and B cells (Eiz-Vesper and Schmetzer, 2020). A primary protective function for long-lived lymphocytes is the ability to distinguish between “self” and “non-self” antigens, such that B and T cells targeting self-antigens are negatively selected during development. The immunological responses to “self” and “non-self” antigens differ between men and women. In general, women have a higher ability to recognize pathogens, recruit more cells of the innate immune system, and develop a better adaptive immune response to pathogens than men (Klein and Flanagan, 2016). Therefore, women are faster at clearing pathogens than men and have better efficacy for vaccines (Giefing-Kroll et al., 2015). Nevertheless, women also have a higher propensity to inflammatory and autoimmune disease susceptibility. In fact, women are four times more likely to develop an autoimmune disease than men, while men are more susceptible to infectious diseases than women (Kronzer et al., 2021; Gay et al., 2021). For the purpose of this review, will focus on the sex-based differences of the effector cells of the adaptive immune system and not innate cells.
Fig. 2.
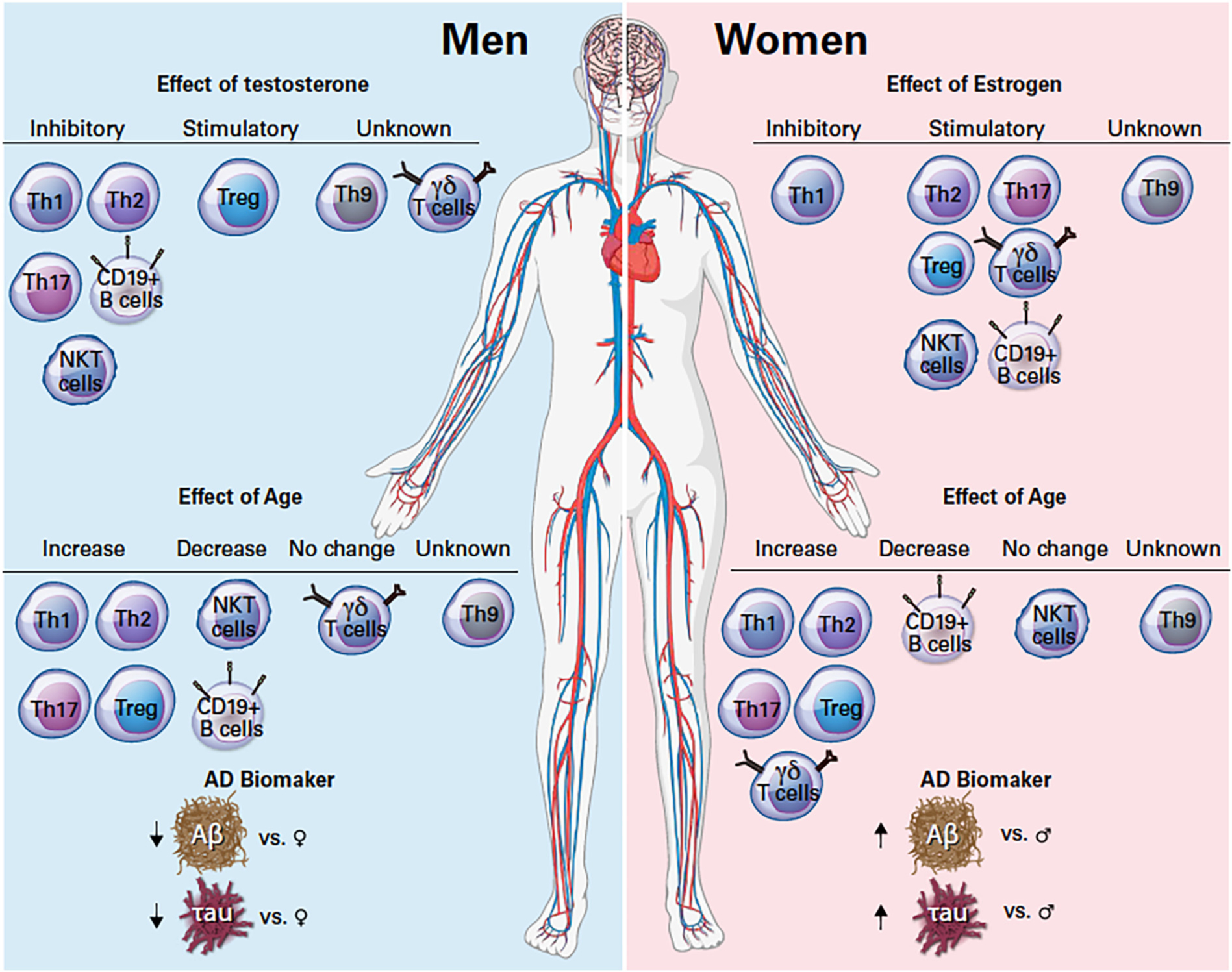
Effect of sex hormones and age on circulating effector immune cells and difference in Alzheimer’s disease biomarker between men and women. Abbreviations: AD: Alzheimer’s disease. Th: Helper T cells, Treg: Regulatory T cells, NKT: Natural Killer T cells, γδ: gamma delta, Aβ: Amyloid beta. Symbols: ↑increase, ↓decrease, ♀ women, ♂ men.
4.1. Sex-based differences in major effector T cell subsets
T helper type 1 (Th1) cells are a subset of CD4+ effector T cells. Th1 cells mediate the immune response against intracellular viral and bacterial pathogens (Berger, 2000). Upon exposure to IL-12, naïve T cells differentiate into Th1 cells, which start to produce interferon gamma (IFNγ), a cytokine that activates macrophages and dendritic cells (DCs). Th1 cells also produce TNF, lymphotoxin-α, and IL-2 which are all involved in the antimicrobial defense (Abbas et al., 1996). Th1 cells display sex-based differences in their response to infection or stimulation. Following a parasitic infection, male mice have elevated levels of Th1-associated IFNγ compared to females (Roberts et al., 2001). After stimulation of human PBMCs with phytohaemagglutinin (PHA), Th1-associated cytokines IFNγ and IL-2 were also higher in younger men of 25 years of age than in women of the same age (Giron-Gonzalez et al., 2000). Age in women especially modulates Th1 responses, as estrogen inhibits the effects of Th1 (Liu et al., 2002; Salem, 2004). Similarly, testosterone also inhibits Th1 differentiation in men (Kissick et al., 2014), suggesting an effect of sex hormones in Th1 responses and their involvement in immunosenescence.
T helper type 2 (Th2) cells mediate the immune response against helminths, venoms, and some bacterial infections by promoting B cell proliferation and immunoglobulin (Ig) isotype class-switching to IgG1 and IgE (Kopf et al., 1993). Th2 cells produce IL-4, IL-5 and IL-13, all cytokines involved in the recruitment of eosinophils, basophils, mast cells, and activation of the “M2” (alternatively activated) macrophages (Abbas et al., 1996). Their response to stimulation also varies between men and women. Human PBMC activation with PHA resulted in higher levels of Th2-associated cytokines in women than in men (Giron-Gonzalez et al., 2000). Estrogen directly increases Th2-associated cytokines, as well as Th2 differentiation. In a study of women during the luteal phase of the menstrual cycle, Faas et al. confirmed that with a high estrogen level during the luteal phase, the Th2 cytokine IL-4 was significantly increased compared to the ovarian cycle when estrogen is low (Faas et al., 2000). Additionally, estrogen increases Th2-associated cytokines IL-4 and IL-13 and Th2 differentiation (Hepworth et al., 2010), and promotes a shift from Th1 to Th2 differentiation (Liu et al., 2002). Interestingly, testosterone inhibits Th2-mediated immune responses (Hepworth et al., 2010). Both testosterone and estrogen decrease with age while Th2 cells increase with age in both men and women, affecting Th2-associated immune response at older ages (Mansfield et al., 2012).
T helper 17 (Th17) cells mediate the immune response against microbial infections, more particularly extracellular bacteria and mucosal fungi, with Th17 activation upregulating the production of IL-17A, IL-17F, IL-21, IL-22 and granulocyte-macrophage colony-stimulating factor (GM-CSF). Th17 effector cytokines promote neutrophil production and recruitment at the site of infection. Th17 cells have been implicated in inflammatory and autoimmune diseases, such as rheumatoid arthritis, with women exhibiting higher prevalence of autoimmune diseases than men (Desai and Brinton, 2019). Stimulated human PBMCs from healthy donors confirmed both higher levels of Th17 cells, and higher IL-17A production in women compared to men (Blanco et al., 2013; Newcomb et al., 2015). In vitro and animal studies both show that estrogen increases IL-17A production and increases Th17 differentiation (Newcomb et al., 2015; Fuseini et al., 2019). On the other hand, the suppressing function of testosterone also applies to Th17; testosterone inhibits Th17 differentiation and therefore inhibits Th17-induced immune responses (Kissick et al., 2014). Th17 cells and associated cytokines increase with age (Bharath et al., 2020), with postmenopausal women exhibiting elevated levels of IL-17A and Th17 cells (Bhadricha et al., 2021). While an increase in Th17 cells is associated with healthy aging (Bharath et al., 2020; Li et al., 2017), a further Th17 increase leads to physiological dysfunctions such as low bone mineral density (Bhadricha et al., 2021). The effect of sex hormones on Th17 dysregulation could suggest their involvement in the development of sex-based vulnerability to different diseases.
T helper 9 (Th9) cells are a more recently identified subset of helper T cells and play an important role in helminth infections, autoimmunity, allergies, and tumor suppression (Anuradha et al., 2016; Pan et al., 2013). TGF-β and IL-4 mediate Th9 cell differentiation. In the presence of TGF-β alone, Th9 cells are differentiated from the switching of Th2 cells (Veldhoen et al., 2008), while the presence of both TGF-β and IL-4 differentiate Th9 cells from naïve T cells. Upon activation, Th9 cells secrete IL-9 to induce early T cell-mediated inflammatory responses (Chen et al., 2019; Gu et al., 2017). IL-9 promotes mast cells proliferation and IgE and IgG production from B cells (Goswami and Kaplan, 2011). Currently, and to the best of our knowledge, there are no studies that have investigated sex-based differences of Th9 cells in either human or animal models, nor the effects of sex hormones on Th9 cells abundance and differentiation that also remains to be elucidated.
Finally, Regulatory T cells (Treg) are a specialized subset of Th cells that maintain immune homeostasis and moderate inflammation in response to pathogens (Vignali et al., 2008). Tregs produce immunosuppressive cytokines: IL-10, transforming growth factor (TGF-β), and IL-35 (Collison et al., 2007). In healthy young individuals between the age of 19 and 26 years old, Treg frequencies were lower in women than in men (Afshan et al., 2012; Singh and Bischoff, 2021), though estrogen regulates Treg differentiation. In fact, estrogen increases FoxP3, a transcription factor that controls Treg development and function, and specifically promotes Treg differentiation (Polanczyk et al., 2004; Tai et al., 2008; Goodman et al., 2020). The promoting effect of estrogen on FoxP3 and Tregs has been attributed, at least in part, to the susceptibility of women to autoimmune diseases such as systemic lupus erythematosus (SLE) (Singh and Bischoff, 2021). Similar to estrogen, testosterone also directly regulates FoxP3 expression and Treg differentiation (Walecki et al., 2015; Fijak et al., 2011), though counterintuitively Treg populations increase with age in both men and women despite the loss of circulating estrogen and testosterone (Huang et al., 2021).
4.2. Sex-based differences in gamma delta T cells
Gamma delta (γδ) T cells are a unique group of T cells that have a γ and δ chain T-Cell Receptor (TCR) while other T cells have an α and β TCR. Unlike the other T cells, γδ activation is major histocompatibility complex (MHC) independent. γδ T cells are more abundant in epithelial and mucosal tissues, and less abundant as circulating cells. Upon activation, γδ T cells produce several proinflammatory cytokines such as IFNγ, TNF, and IL-17 (Carding and Egan, 2002). Sex-based differences studies in γδ T cells are limited. Although, Caccamo et al. report that γδ T cells are higher in women than in men (Caccamo et al., 2006), they also reported that during the early years of life, γδ T cells were similar. The difference between the sexes manifested at puberty, which suggests an effect of estrogen in the abundance of γδ T cells (Caccamo et al., 2006). In animal models, estrogen has been shown to increase γδ T cells in the uterus (Kang et al., 2022) and lymph nodes, but inhibits this population in the joints (Andersson et al., 2015).
4.3. Sex-based differences in NK T cells
Natural Killer T (NKT) cells are innate-like lymphocytes that share characteristics of both the innate and adaptive immune systems. NKT cells express semi-invariant T cell receptors that recognize glycolipids associated with the CD1d molecule, which is detrimental for NKT cell development (Bendelac et al., 1997). NKT cells produce cytokines also produced by other Th cell subsets such as IL-4 from Th2 cells and IFNγ from Th1 cells (Coquet et al., 2008). NKT cells are found in various tissues including the thymus, spleen, and bone marrow, and are in very low abundance in peripheral blood and lymph nodes. IL-15 regulates NKT cell homeostasis and maintenance in the periphery and TGF-β promotes the conversion of Th cells into NKT cells (Li and Flavell, 2008) (Ranson et al., 2003). NKT cells are more abundant in women than in men, and estrogen promotes IFNγ production from NKT cells while testosterone inhibits IFNγ production (Gourdy et al., 2005; Lotter et al., 2013). This sex difference in NKT populations is more prominent over 60 years of age (Sandberg et al., 2003; Montoya et al., 2007; Kee et al., 2012; Bernin et al., 2016; Molling et al., 2005) and supports a differential immune response between women and men for this subpopulation.
4.4. Sex-based differences in B cells
B cells, also known as B lymphocytes, are part of the adaptive immune system and are the regulators of the humoral immune system. The humoral immune system is a function of B cells and a part of the adaptive immune response responsible for the production of antibodies or immunoglobulins (Ig) (Janeway et al., 2005). In addition to producing immunoglobulins, B cells also act as APCs to activate T cells, while other APCs activate B cells to become memory B cells (Chen and Jensen, 2008). Additionally, B cells produce proinflammatory cytokines such as IL-6, TNF, and lymphotoxin-α. B cells activation and differentiation occur in either a T cell-dependent or independent manner; the type of activation depends on the type of antigen encountered. B cell differentiation generates different B cells subsets that produce different Ig(s) (Doherty et al., 2018), modulating the effector function based on subsets.
Naïve B cells are poor APCs, but they can still induce naïve T cell proliferation (Gunzer et al., 2004; Reichardt et al., 2007). After interaction with antigens through the B cell antigen receptors (BCR) in secondary lymphoid organs, naïve B cells can differentiate into germinal center B cells, short-lived plasma cells, or memory B cells. Naïve B cells express IgM which has low affinity to antigens. Upon activation, IgM and IgD transform to IgG, IgA, or IgE (Shi et al., 2019). Memory B cells are long-lived quiescent cells though fast-acting to antigens once “experienced”. Memory B cells proliferate in response to antigen and remain in a sustained resting state until a rapid response is needed upon subsequent presentation of the antigen (Shlomchik and Weisel, 2012). Memory B cells consist of high affinity and class-switching antibodies. There are several types of memory B cells that generate antibodies: IgM+, IgG+, IgA+ and IgE+ memory B cells. IgG+ memory B cells tend to differentiate into plasma cells (Kurosaki et al., 2015), while plasmablasts derive from naïve B cells in the secondary lymphoid organs; these cells differentiate into non-dividing or short-lived plasma cells (Nutt et al., 2015). Plasma cells are terminally differentiated, long-lived B cells can generate from germinal center B cells in a T cell-dependent or independent mechanism. They do not need to be activated by antigens to produce antibodies (Nutt et al., 2015), which include a variety of Igs, though IgG is the most expressed.
Regulatory B cells (Bregs) have a main function to maintain immune tolerance and reestablish immune homeostasis. Much like Tregs, Bregs act as immunosuppressants. Bregs achieve their immunosuppressive function by inhibiting the action of effector T cells such as Th1 and Th17, by both inhibiting Th1 and Th17 differentiation and enhancing Treg differentiation (Kessel et al., 2012). Activation of Bregs triggered by an increase in pro-inflammatory cytokines leads to the production of IL-10, TGF-β, and IL-35 from Bregs to suppress local inflammation. Bregs possess a heterogenous population which has made it challenging to identify regulatory markers, including transcription factors, with one hypothesis suggesting that Bregs are not derived from a specific lineage but are reactive from all potential B cells lineages (Rosser and Mauri, 2015).
Studies on sex differences of B cells or the effect of sex hormones on B cells have focused on CD19+ B cells, which encompasses all B cells subsets and not the different subsets of B cells described above. Identified sex differences in humoral immunity is thus far very limited, with naïve (CD5+) B cells higher in males than females in the first 3 years of life (Lundell et al., 2015). In vitro studies of human PBMCs show a higher level of B cells in females (Abdullah et al., 2012; Teixeira et al., 2011) and females also have higher circulating basal Igs than males, which contributes to the greater antibody responses. This includes higher levels of immunoglobulin in response to vaccines and infections for females (Furman et al., 2014; Klein et al., 2010). Sex hormones have the opposite effects on B cell regulation as estrogen inhibits B cells lymphopoiesis, activates B cells function, and increases Igs production (Masuzawa et al., 1994; Kanda and Tamaki, 1999). On the other hand, testosterone inhibits the humoral immune response mainly by inhibiting Igs production (Kanda et al., 1996). Aging sees a decline in circulating B cells and an impaired antibody response (de Mol et al., 2021). Both males and females exhibit decreased B cells with aging, but this decrease is more accelerated in males than it is in females. While an earlier decrease in B cells in women is associated with decreased estrogen after menopause, there is a greater decline in B cells in men after the age of 75 (Klein and Flanagan, 2016; Teixeira et al., 2011).
5. Adaptive immune system in Alzheimer’s disease and related dementia
The role of innate immune cells, including microglia, in the pathogenesis of ADRD has been extensively studied (Lee et al., 2021). However, the role of adaptive immune cells remains in its infancy. Table 1 summarizes the changes observed in effector immune cells of the adaptive immune system. In 1988, Rogers et al. first identified an increase in CD4+ T cells (i.e., general Th cells populations) and cytotoxic CD8+ T cells in post-mortem AD patients’ brains compared to healthy controls (Rogers et al., 1988). In 1993, the association of B cells and AD was first introduced when circulating B cells from AD patients were found to secrete antibodies against Aβ (Γασκιν ετ αλ., 1993). Since these first observations of T and B cells in AD, there has been increased interest in the role of adaptive immune cells in the pathogenesis of ADRD. Numerous studies since have confirmed CD4+ T cells in post-mortem AD brain, particularly in areas of neuritic plaques, and major histocompatibility complex (MHC) II+ microglia which act as APCs to activate CD4+ T cells (McGeer et al., 1989; Togo et al., 2002; Parachikova et al., 2007). CSF-derived studies show that CD4+ T cells levels are similar between AD patients and healthy controls, though reduced in patients with aMCI which correlated with increased Aβ deposition in the brain (Joshi et al., 2022; Stowe et al., 2017). In contrast, other studies found an increase in CD4+ T cells, and particularly activated CD4+ T cells in the CSF of MCI and AD patients (Liu et al., 2018; Lueg et al., 2015; Saresella et al., 2011). The difference between these two groups could be attributed to the number of patients included in those studies, as studies that included larger numbers in either the MCI or AD group had an increase in CD4+ T cell presentation.
Table 1.
Effector immune cells of the adaptive immune system levels in ADRD.
| Cell Type | effector cells’ changes in ADRD |
|---|---|
| Th1 | ⇔ in MCI due to AD (Oberstein et al., 2018) ⇑ in AD (Zhang and Niu, 2022) |
| Th2 | ⇓ in AD (Zhang and Niu, 2022) |
| Th17 | ⇑ in MCI due to AD (Oberstein et al., 2018) ⇑ in AD (Zhang and Niu, 2022), (Zeng et al., 2022) Through IL-17A -⇑ in aMCI (Dubenko et al., 2021) -⇑ in AD (Chen et al., 2014), (Behairi et al., 2015), (Dubenko et al., 2021) |
| Treg | ⇑ in MCI (Fu et al., 2020) ⇓ in AD (Ciccocioppo et al., 2019), (Fu et al., 2020) ⇔ in MCI and AD (Oberstein et al., 2018) |
| Th9 | Through IL-9 ⇑ in MCI (Saresella et al., 2011) ⇑ in AD (Saresella et al., 2011) |
| γδ T cells | Unknown in human |
| NKT | ⇑ in MCI and AD (Busse et al., 2021) |
| Memory B cells | ⇑ in MCI (Stowe et al., 2017) ⇑ in AD (Bulati et al., 2015), (Park et al., 2022) ⇔ in AD (Söllvander, 2015) |
| Plasma B cells | ⇓in AD (Pellicanò, 2010), (Busse et al., 2017) |
Abbreviations: MCI: Mild Cognitive Impairment, AD: Alzheimer’s Disease, Th: Helper T cells, Treg: Regulatory T cells, NKT: Natural Killer T cells, γδ: gamma delta. Symbols: ⇑ increase, ⇓ decrease and ⇔ no change.
Besides the brain/CSF compartments, numerous studies have also explored the role of circulating effector cells in ADRD. Circulating CD4+ T cells are higher in ADRD patients than in cognitively normal subjects (Larbi et al., 2009). Of the aforementioned CD4+ T cells subsets, Oberstein et al. showed that circulating Th17 cells were higher in subjects with MCI due to AD than either cognitively normal subjects or those with non-AD MCI, concomitant with no change in circulating Th1 cells (Oberstein et al., 2018). While this study did not find any difference in Th1 cells, Zhang et al. reported that circulating Th1 cells were higher in subjects with AD (Zhang and Niu, 2022). On the other hand, Th2 cells and Th2-associated molecules such as monocytes chemotactic protein-1 (MCP-1; CCL2) and IL-4 were reported to be lower in AD subjects (Zhang and Niu, 2022; Reale et al., 2006). This suggests that Th1-mediated inflammatory responses may go unchecked by the anti-inflammatory effect of Th2, with a Th1/Th2 imbalance in ADRD. Other studies show pathology related instead to Th17 cells, with higher circulating Th17 populations in AD subjects (Zhang and Niu, 2022; Zeng et al., 2022). Th17’s implication in ADRD has been highly assessed through quantification of plasma levels of IL-17A, with several studies reporting that circulating IL-17A is increased in aMCI and AD subjects (Chen et al., 2014; Behairi et al., 2015; Dubenko et al., 2021). Tregs are also lower in patients with AD (Ciccocioppo et al., 2019), which is the anti-inflammatory counter population to Th17 cells. Fu et al. reported a low level of Tregs cells in AD subjects however in subjects with MCI, prodromal for AD, Tregs cells were high compared to healthy control and AD subjects (Fu et al., 2020). A different study reported that there was no difference in Tregs between MCI, AD, and healthy patients, but Treg positively correlated with total τau and pTau181 in AD subjects and also identified an imbalance between Th17 and Tregs in AD patients (Oberstein et al., 2018).
While there is a rise in interest in effector cells in the ADRD, circulating NKT cells, γδ T cells, and Th9 cells are minimally explored, with only one study from Busse et al. finding that NKT cells are increased in the CSF of MCI and AD subjects (Busse et al., 2021). The only human study on γδ T cells looked at the T-cell receptor γ genes (TRGs) in PBMCs of AD subjects but not at γδ T cell population levels. Nevertheless, looking at the TRG repertoire, they found an AD-associated TRG pattern in AD patients (Aliseychik et al., 2020), suggesting an altered function or implication of γδ T cells in ADRD. An early study in Th9 cells found an increase in Th9 cells and IL-9 cytokines in patients with AD (Saresella et al., 2011). However, a most recent study found that IL-9 was increased in AD only in African Americans and not in Caucasians (Wharton et al., 2019). These findings raise a challenge to investigate the role of Th9 in ADRD due to differences between Caucasians and African-Americans and suggest race and ethnicity as another risk factor in the development and pathology of ADRD, which is not discussed in this review. All of these initial studies cast more confusion as to the understanding of the T cell response in the development of AD, particularly in the prodromal stage. It is of obvious importance that future studies should include longitudinal studies, within-patient immunophenotyping concomitant with ADRD biomarkers, and imaging quantification to identify the critically relevant subsets for dementia.
B cells in ADRD are also understudied. Although, there is evidence supporting the involvement of B cells in the development of AD (Plantone et al., 2022), B cell contribution in AD pathogenesis is still unclear and the results are contradictory. In subjects with MCI, memory B cells are increased in the CSF, which positively correlated with Aβ deposition in the brain and exacerbated in APOε4 carriers (Stowe et al., 2017). IgG+IgD−CD27− memory B cells in the circulation were also increased in patients with moderate to severe AD (Bulati et al., 2015). A more recent study confirmed that B cell populations and IgG were increased in AD patients, and levels correlated with Aβ deposition in the brain most likely due to microglial loss-of-function and impaired Aβ clearance (Park et al., 2022). In contrast, plasma B cells were decreased in subjects with MCI and AD compared to healthy controls, though this also associated with an increase in Aβ deposition in the brain (Stowe et al., 2017; Pellicanò, 2010; Busse et al., 2017). This suggests that memory B cells subsets are altered in ADRD patients, though as both increased and decreased levels associated with Aβ deposition it is difficult to know whether there is a direct contribution to the ongoing amyloid deposition. Additionally, an immortalized B cell line from an AD patient was shown to overproduce TNF and induce Aβ plaque formation in vitro (Dezfulian, 2018) suggesting a direct mechanism contributing to amyloid burden. Another study, however, found no difference in circulating B cells between AD and healthy groups, though the AD group had an increase in anti-Aβ antibodies (Söllvander, 2015). Ultimately, the differences in circulating T and B lymphocytes in AD subjects suggest a dysregulation in adaptive immune function.
Beside their altered levels, effector cells of the adaptive immune system have been associated with preventing Aβ clearance from the brain, exacerbating subsequent accumulation, and hyperphosphorylation of the τau protein. These roles of effector cells in the pathogenesis of AD have mostly been studied in animal models of AD. In AD models, Th1 cells through IFNγ production, as well as Th17 cells through IL-17A production, are associated with increased microglial activation, Aβ accumulation in the brain, and neuroinflammation, while immunization against Aβ1–42 reduces Aβ accumulation in the brain (Browne et al., 2013; Machhi et al., 2021; Town et al., 2002; Monsonego et al., 2003). This latter result is secondary to immune switching from Th1 responses to increased Th2 responses. In addition to its association with Aβ accumulation in the brain, Th17 cells are also associated with neurodegeneration in rats and APP/PS1 mice (Zhang et al., 2013; Wang et al., 2019), and synaptic dysfunction in APP/PS1 mice (Liu et al., 2021), while IL-17A-producing γδ T cells were involved in memory deficits in 3xTg-AD mice (Brigas et al., 2021). Inhibition of IL-17A prevented cognitive decline, synaptic dysfunction, and Aβ accumulation in these models (Liu et al., 2021; Brigas et al., 2021). In AD animal models, activated B cells expressing IL-6, IL-10, IFNγ, and TGFβ were increased in 3xTg-AD and APP/PS1 mice, with B cells depletion reversing the progression of AD (Kim et al., 2021). While the role of the dysregulation in adaptive immunity is suggested, there is still the need to understand the mechanisms associated with adaptive immune dysregulation and determine whether these cells and their cytokines are the cause or consequence of neurodegeneration, and - importantly - whether they differ between men and women as they age.
6. Sex differences, Alzheimer’s disease and related dementia, and precision medicine
Despite tremendous progress in AD research, the complexity of AD pathophysiology remains unsolved. Recent years have seen many improvements in the diagnosis of ADRD, from the detection of changes in brain function and structures through neuroimaging to the identification of circulating biomarkers that reflect ADRD-related changes in the brain (Turner et al., 2020). However, there is still not an efficacious therapeutic target for AD, except for those associated with the symptoms of AD, and clinical trials of ADRD have failed to deliver promising results (Asher and Priefer, 2022). In fact, over the past several decades, various clinical trials with different approaches and targets struggled to deliver positive outcomes, with those targets mostly focused on Aβ synthesis and clearance (Asher and Priefer, 2022; Huang et al., 2020; Song et al., 2022). One popular approach was the anti-Aβ vaccination approach. Unfortunately, those trials were terminated as some participants developed meningoencephalitis or worsening of the cognitive function (Song et al., 2022). Other reasons for AD trial terminations include worsening of outcome, death, unable to reach the primary endpoint, and no change in Aβ clearance. Of note, these failures may be due to the complexity of ADRD pathology, with no clear mechanism of neurodegeneration caused by Aβ deposition and accumulation in the brain. Nevertheless, recent trials on humanized IgG1 monoclonal antibodies against Aβ brought about a glimmer of hope among those in the early stages of AD. Recently, conditionally-approved Lecanemab was shown to decrease Aβ in the brain and delay cognitive decline in subjects in the early stages of AD in phase 3 clinical trial (NCT03887455) (van Dyck et al., 2023). The conditional approval is the second conditional approval, the first being Aducanumab based on the ability to decrease Aβ and slow cognitive decline (NCT02484547) (Nistico and Borg, 2021; Day et al., 2022). However, the changes seen in both drugs are relatively modest compared to the control group. Additionally, both drugs had incidences of ARIA-E or cerebral edema, but Lecanemab had the lowest incidence and is more promising (Shi et al., 2022). Therefore, both drugs are required to undergo additional trials (NCT04241068, NCT04468659) to test their safety and efficacy.
Other trials (NCT00164749, NCT00099710) focused on inflammation as a target but were also ineffective. Unfortunately, the majority of these clinical trials also failed to report on sex-based differences in their findings, although they typically report the percentage of women and men. A few studies have included sex-specific analyses, but only 12.5% of these studies have reported their results based on sex-stratified analyses (Martinkova et al., 2021). The most recent years have seen clinical trials that include sex differences in their analyses, with a fortunate push in preclinical research to also include both females and males in animal studies for ADRD, as well as other diseases of aging. However, only a few of those animal studies have reported sex differences in outcome measures, or are powered a priori to achieve this end (Waters et al., 2021).
With regard to precision medicine, several recent studies using the Comparative Effectiveness Dementia & Alzheimer’s Registry (CEDAR) approach showed positive outcomes in improving cognition for a more individualized approach in clinical management targeting different risk factors of ADRD, including cardiovascular diseases (Isaacson et al., 2019; Saif et al., 2022; Isaacson et al., 2018). Saif et al. evaluated the efficiency of sex-based differences in an individualized clinical management of AD and found that the incidence of dementia was much improved in women versus men (Saif et al., 2022). Additionally, women also showed a better improvement in the amelioration of cardiovascular risk factors as critical risk factors for ADRD (Saif et al., 2022). However, the sample size included in this study was relatively small and a larger sample size would be more beneficial in drawing definitive sex-based conclusions. Nevertheless, besides the known risk factors of ADRD, the rise of adaptive immunity in ADRD brings in a new player to consider in the quest to improve precision medicine and should thus be included as a longitudinal biomarker and/or outcome as there is a dearth of knowledge of within-patient adaptive immune changes with the progression of ADRD.
7. Conclusion
There is mounting evidence that supports sex-based differences in the development of ADRD, including the incidence rate of ADRD being higher in women, the presentation of those conditions, and overall disease progression. The risk factors associated with AD also have sex differences: 1) age, women live longer than men, 2) cardiovascular diseases, 3) metabolic diseases, 4) genetic factors, which were not discussed here but are discussed in the following review (Dumitrescu et al., 2019b), and 5) sex is itself a risk factor of ADRD. Moreover, there is also the inflammatory risk factor of ADRD that differs between women and men. Yet, sex differences in most studies are still lacking, as is a holistic approach to studying the adaptive immune system during ADRD. While there has been a new focus and interest in the inclusion of sex differences in ADRD in recent years, it is of great importance that sex differences be included in both animal and human studies for further advancement of the field that will lead to a more precise and targeted screening and treatment for the predominant global health burden within the next 50 years.
Acknowledgments
We would like to thank Matthew Hazzard and Thomas Dolan, University of Kentucky, for creating the medical illustrations. This work has been supported by the University of Kentucky Lyman T. Johnson postdoctoral fellowship to JL, National Institute of Aging R56 AG074613 to AS, the University of Kentucky Alzheimer’s Disease research center, National Institute of Neurological Disorders and Stroke awards R21 NS104509 and R01 NS102417 to NM supported this work. This project was also supported by the Texas Alzheimer’s Research and Care Consortium (TARCC).
Footnotes
CRediT authorship contribution statement
Jenny Lutshumba: Conceptualization, Writing – original draft, Visualization, Funding acquisition. Donna Wilcock: Conceptualization, Writing – review & editing. Nancy L. Monson: Conceptualization, Writing – review & editing, Funding acquisition. Ann M. Stowe: Conceptualization, Writing – review & editing, Funding acquisition, Supervision.
Data availability
No data was used for the research described in the article.
References
- Abbas AK, Murphy KM, Sher A, 1996. Functional diversity of helper T lymphocytes. Nature 383 (6603), 787–793. [DOI] [PubMed] [Google Scholar]
- Abdullah M, et al. , 2012. Gender effect on in vitro lymphocyte subset levels of healthy individuals. Cell. Immunol 272 (2), 214–219. [DOI] [PubMed] [Google Scholar]
- Afshan G, Afzal N, Qureshi S, 2012. CD4+CD25(hi) regulatory T cells in healthy males and females mediate gender difference in the prevalence of autoimmune diseases. Clin. Lab 58 (5–6), 567–571. [PubMed] [Google Scholar]
- Ahlbom E, Prins GS, Ceccatelli S, 2001. Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Res. 892 (2), 255–262. [DOI] [PubMed] [Google Scholar]
- Aliseychik M, et al. , 2020. Dissection of the human T-cell receptor gamma gene repertoire in the brain and peripheral blood identifies age- and Alzheimer’s disease-associated clonotype profiles. Front. Immunol 11, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann A, et al. , 2014. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann. Neurol 75 (4), 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-de-la-Rosa M, et al. , 2005. Estradiol prevents neural tau hyperphosphorylation characteristic of Alzheimer’s disease. Ann. N. Y. Acad. Sci 1052, 210–224. [DOI] [PubMed] [Google Scholar]
- 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 18 (4), 2022, 700–789. [DOI] [PubMed] [Google Scholar]
- Andersson S, Bishop RW, Russell DW, 1989. Expression cloning and regulation of steroid 5 alpha-reductase, an enzyme essential for male sexual differentiation. J. Biol. Chem 264 (27), 16249–16255. [PMC free article] [PubMed] [Google Scholar]
- Andersson A, et al. , 2015. IL-17-producing gammadeltaT cells are regulated by estrogen during development of experimental arthritis. Clin. Immunol 161 (2), 324–332. [DOI] [PubMed] [Google Scholar]
- Anuradha R, et al. , 2016. IL-10- and TGFbeta-mediated Th9 responses in a human helminth infection. PLoS Negl. Trop. Dis 10 (1), e0004317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher S, Priefer R, 2022. Alzheimer’s disease failed clinical trials. Life Sci. 306, 120861. [DOI] [PubMed] [Google Scholar]
- Atallah A, Mhaouty-Kodja S, Grange-Messent V, 2017. Chronic depletion of gonadal testosterone leads to blood-brain barrier dysfunction and inflammation in male mice. J. Cereb. Blood Flow Metab 37 (9), 3161–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azcoitia I, et al. , 2001. Brain aromatase is neuroprotective. J. Neurobiol 47 (4), 318–329. [DOI] [PubMed] [Google Scholar]
- Babapour Mofrad R, et al. , 2020. Sex differences in CSF biomarkers vary by Alzheimer disease stage and APOE epsilon4 genotype. Neurology 95 (17), e2378–e2388. [DOI] [PubMed] [Google Scholar]
- Barnes LL, et al. , 2005. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch. Gen. Psychiatry 62 (6), 685–691. [DOI] [PubMed] [Google Scholar]
- Baumgartner RN, et al. , 1999. Predictors of skeletal muscle mass in elderly men and women. Mech. Ageing Dev 107 (2), 123–136. [DOI] [PubMed] [Google Scholar]
- Beecham GW, et al. , 2010. PCDH11X variation is not associated with late-onset Alzheimer disease susceptibility. Psychiatr. Genet 20 (6), 321–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behairi N, et al. , 2015. All-trans-retinoic acid modulates nitric oxide and interleukin17A production by peripheral blood mononuclear cells from patients with Alzheimer’s disease. Neuroimmunomodulation 22 (6), 385–393. [DOI] [PubMed] [Google Scholar]
- Bellenguez C, et al. , 2022. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat. Genet 54 (4), 412–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendelac A, et al. , 1997. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu. Rev. Immunol 15, 535–562. [DOI] [PubMed] [Google Scholar]
- Benedictus MR, et al. , 2015. White matter hyperintensities relate to clinical progression in subjective cognitive decline. Stroke 46 (9), 2661–2664. [DOI] [PubMed] [Google Scholar]
- Berger A, 2000. Th1 and Th2 responses: what are they? BMJ 321 (7258), 424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernin H, et al. , 2016. The cytokine profile of human NKT cells and PBMCs is dependent on donor sex and stimulus. Med. Microbiol. Immunol 205 (4), 321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhadricha H, et al. , 2021. Increased frequency of Th17 cells and IL-17 levels are associated with low bone mineral density in postmenopausal women. Sci. Rep 11 (1), 16155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharath LP, et al. , 2020. Metformin enhances autophagy and normalizes mitochondrial function to alleviate aging-associated inflammation. Cell Metab. 32 (1), 44–55 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco LP, et al. , 2013. Gender-biased regulation of human IL-17-producing cells in vitro by peptides corresponding to distinct HLA-DRB1 allele-coded sequences. J. Immune Based Ther. Vaccines Antimicrob 2 (3), 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanken AE, Nation DA, 2020. Does gender influence the relationship between high blood pressure and dementia? Highlighting areas for further investigation. J. Alzheimers Dis 78 (1), 23–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigas HC, et al. , 2021. IL-17 triggers the onset of cognitive and synaptic deficits in early stages of Alzheimer’s disease. Cell Rep. 36 (9), 109574. [DOI] [PubMed] [Google Scholar]
- Browne TC, et al. , 2013. IFN-gamma Production by amyloid beta-specific Th1 cells promotes microglial activation and increases plaque burden in a mouse model of Alzheimer’s disease. J. Immunol 190 (5), 2241–2251. [DOI] [PubMed] [Google Scholar]
- Buckley RF, et al. , 2018. Sex, amyloid, and APOE epsilon4 and risk of cognitive decline in preclinical Alzheimer’s disease: findings from three well-characterized cohorts. Alzheimers Dement. 14 (9), 1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulati M, et al. , 2015. Double negative (IgG+IgD-CD27-) B cells are increased in a cohort of moderate-severe Alzheimer’s disease patients and show a proinflammatory trafficking receptor phenotype. J. Alzheimers Dis 44 (4), 1241–1251. [DOI] [PubMed] [Google Scholar]
- Burke SL, et al. , 2019. Sex differences in the development of mild cognitive impairment and probable Alzheimer’s disease as predicted by hippocampal volume or white matter hyperintensities. J. Women Aging 31 (2), 140–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse M, et al. , 2017. Alterations in the peripheral immune system in dementia. J. Alzheimers Dis 58 (4), 1303–1313. [DOI] [PubMed] [Google Scholar]
- Busse S, et al. , 2021. Dementia-associated changes of immune cell composition within the cerebrospinal fluid. Brain Behav. Immun. Health 14, 100218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo N, et al. , 2006. Sex-specific phenotypical and functional differences in peripheral human Vgamma9/Vdelta2 T cells. J. Leukoc. Biol 79 (4), 663–666. [DOI] [PubMed] [Google Scholar]
- Caceres A, et al. , 2020. Extreme downregulation of chromosome Y and Alzheimer’s disease in men. Neurobiol. Aging 90, 150 e1–150 e4. [DOI] [PubMed] [Google Scholar]
- Cano RLE, Lopera HDE, 2013. Introduction to T and B lymphocytes. In: Autoimmunity: From Bench to Bedside [Internet]. El Rosario: University Press. [PubMed] [Google Scholar]
- Carding SR, Egan PJ, 2002. Gammadelta T cells: functional plasticity and heterogeneity. Nat. Rev. Immunol 2 (5), 336–345. [DOI] [PubMed] [Google Scholar]
- Carrasquillo MM, et al. , 2009. Genetic variation in PCDH11X is associated with susceptibility to late-onset Alzheimer’s disease. Nat. Genet 41 (2), 192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JC, et al. , 2007. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J. Neurosci 27 (48), 13357–13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, 2022. Life Expectancy in the U.S. Dropped for the Second Year in a Row in 2021.
- Charlesworth B, 2003. The organization and evolution of the human Y chromosome. Genome Biol. 4 (9), 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Jensen PE, 2008. The role of B lymphocytes as antigen-presenting cells. Arch. Immunol. Ther. Exp 56 (2), 77–83. [DOI] [PubMed] [Google Scholar]
- Chen JM, et al. , 2014. Increased serum levels of interleukin-18, −23 and −17 in Chinese patients with Alzheimer’s disease. Dement. Geriatr. Cogn. Disord 38 (5–6), 321–329. [DOI] [PubMed] [Google Scholar]
- Chen J, et al. , 2019. T Helper 9 cells: a new player in immune-related diseases. DNA Cell Biol. 38 (10), 1040–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrier MM, Aubin S, Higano CS, 2009. Cognitive and mood changes in men undergoing intermittent combined androgen blockade for non-metastatic prostate cancer. Psychooncology 18 (3), 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrier MM, et al. , 2015. Testosterone treatment of men with mild cognitive impairment and low testosterone levels. Am. J. Alzheimers Dis. Other Dement 30 (4), 421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HM, Kim HC, Kang DR, 2017. Sex differences in hypertension prevalence and control: Analysis of the 2010–2014 Korea National Health and Nutrition Examination Survey. PLoS One 12 (5), e0178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo F, et al. , 2019. The characterization of regulatory T-cell profiles in Alzheimer’s disease and multiple sclerosis. Sci. Rep 9 (1), 8788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collison LW, et al. , 2007. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 450 (7169), 566–569. [DOI] [PubMed] [Google Scholar]
- Coquet JM, et al. , 2008. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proc. Natl. Acad. Sci. U. S. A 105 (32), 11287–11292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, et al. , 2004. The biphasic relationship between regional brain senile plaque and neurofibrillary tangle distributions: modification by age, sex, and APOE polymorphism. Ann. N. Y. Acad. Sci 1019, 24–28. [DOI] [PubMed] [Google Scholar]
- Craig MC, Maki PM, Murphy DG, 2005. The Women’s Health Initiative Memory Study: findings and implications for treatment. Lancet Neurol. 4 (3), 190–194. [DOI] [PubMed] [Google Scholar]
- Cui J, Shen Y, Li R, 2013. Estrogen synthesis and signaling pathways during aging: from periphery to brain. Trends Mol. Med 19 (3), 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SR, Wahlin-Jacobsen S, 2015. Testosterone in women–the clinical significance. Lancet Diabetes Endocrinol. 3 (12), 980–992. [DOI] [PubMed] [Google Scholar]
- Davis EJ, et al. , 2020. A second X chromosome contributes to resilience in a mouse model of Alzheimer’s disease. Sci. Transl. Med 12 (558). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EJ, et al. , 2021. Sex-specific association of the X chromosome with cognitive change and tau pathology in aging and Alzheimer disease. JAMA Neurol. 78 (10), 1249–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day GS, et al. , 2022. Aducanumab use in symptomatic Alzheimer disease evidence in focus: a report of the AAN Guidelines Subcommittee. Neurology 98 (15), 619–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw FE, et al. , 2001. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J. Neurol. Neurosurg. Psychiatry 70 (1), 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mol J, et al. , 2021. The dynamics of B cell aging in health and disease. Front. Immunol 12, 733566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai MK, Brinton RD, 2019. Autoimmune disease in women: endocrine transition and risk across the lifespan. Front. Endocrinol. (Lausanne) 10, 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezfulian M, 2018. A new Alzheimer’s disease cell model using B cells to induce beta amyloid plaque formation and increase TNF alpha expression. Int. Immunopharmacol 59, 106–112. [DOI] [PubMed] [Google Scholar]
- Doherty DG, et al. , 2018. Activation and regulation of B cell responses by invariant natural killer T cells. Front. Immunol 9, 1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, et al. , 2022. Sex differences in the association between cardiovascular diseases and dementia subtypes: a prospective analysis of 464,616 UK Biobank participants. Biol. Sex Differ 13 (1), 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubal DB, et al. , 2001. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc. Natl. Acad. Sci. U. S. A 98 (4), 1952–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubenko OE, Chyniak OS, Potapov OO, 2021. Levels of proinflammatory cytokines Il-17 and Il-23 in patients with Alzheimer’s disease, mild cognitive impairment and vascular dementia. Wiad. Lek 74 (1), 68–71. [PubMed] [Google Scholar]
- Dumanski JP, et al. , 2016. Mosaic loss of chromosome Y in blood is associated with Alzheimer disease. Am. J. Hum. Genet 98 (6), 1208–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumanski JP, et al. , 2021. Immune cells lacking Y chromosome show dysregulation of autosomal gene expression. Cell. Mol. Life Sci 78 (8), 4019–4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrescu L, et al. , 2019a. Sex differences in the genetic predictors of Alzheimer’s pathology. Brain 142 (9), 2581–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrescu L, et al. , 2019b. Sex differences in the genetic architecture of Alzheimer’s disease. Curr. Genet. Med. Rep 7 (1), 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiz-Vesper B, Schmetzer HM, 2020. Antigen-presenting cells: potential of proven und new players in immune therapies. Transfus. Med. Hemother 47 (6), 429–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faas M, et al. , 2000. The immune response during the luteal phase of the ovarian cycle: a Th2-type response? Fertil. Steril 74 (5), 1008–1013. [DOI] [PubMed] [Google Scholar]
- Fatemi F, et al. , 2018. Sex differences in cerebrovascular pathologies on FLAIR in cognitively unimpaired elderly. Neurology 90 (6), e466–e473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fijak M, et al. , 2011. Testosterone replacement effectively inhibits the development of experimental autoimmune orchitis in rats: evidence for a direct role of testosterone on regulatory T cell expansion. J. Immunol 186 (9), 5162–5172. [DOI] [PubMed] [Google Scholar]
- Fisk DM, 2003. American Labor in the 20th Century.
- Ford AH, et al. , 2018. Sex hormones and incident dementia in older men: the health in men study. Psychoneuroendocrinology 98, 139–147. [DOI] [PubMed] [Google Scholar]
- Forsberg LA, Gisselsson D, Dumanski JP, 2017. Mosaicism in health and disease -clones picking up speed. Nat. Rev. Genet 18 (2), 128–142. [DOI] [PubMed] [Google Scholar]
- Fu J, et al. , 2020. Mild cognitive impairment patients have higher regulatory T-Cell proportions compared with Alzheimer’s disease-related dementia patients. Front. Aging Neurosci 12, 624304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman D, et al. , 2014. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc. Natl. Acad. Sci. U. S. A 111 (2), 869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuseini H, et al. , 2019. ERalpha signaling increased IL-17A production in Th17 cells by upregulating IL-23R expression, mitochondrial respiration, and proliferation. Front. Immunol 10, 2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamberger D, et al. , 2017. Identification of clusters of rapid and slow decliners among subjects at risk for Alzheimer’s disease. Sci. Rep 7 (1), 6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskin F, et al. , 1993. Human antibodies reactive with beta-amyloid protein in Alzheimer’s disease. J. Exp. Med 177 (4), 1181–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay L, et al. , 2021. Sexual dimorphism and gender in infectious diseases. Front. Immunol 12, 698121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giefing-Kroll C, et al. , 2015. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell 14 (3), 309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giron-Gonzalez JA, et al. , 2000. Consistent production of a higher TH1:TH2 cytokine ratio by stimulated T cells in men compared with women. Eur. J. Endocrinol 143 (1), 31–36. [DOI] [PubMed] [Google Scholar]
- Goodman WA, et al. , 2020. Impaired estrogen signaling underlies regulatory T cell loss-of-function in the chronically inflamed intestine. Proc. Natl. Acad. Sci. U. S. A 117 (29), 17166–17176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami R, Kaplan MH, 2011. A brief history of IL-9. J. Immunol 186 (6), 3283–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourdy P, et al. , 2005. Relevance of sexual dimorphism to regulatory T cells: estradiol promotes IFN-gamma production by invariant natural killer T cells. Blood 105 (6), 2415–2420. [DOI] [PubMed] [Google Scholar]
- Grimm A, et al. , 2014. Improvement of neuronal bioenergetics by neurosteroids: implications for age-related neurodegenerative disorders. Biochim. Biophys. Acta 1842 (12 Pt A), 2427–2438. [DOI] [PubMed] [Google Scholar]
- Gu ZW, Wang YX, Cao ZW, 2017. Neutralization of interleukin-17 suppresses allergic rhinitis symptoms by downregulating Th2 and Th17 responses and upregulating the Treg response. Oncotarget 8 (14), 22361–22369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunzer M, et al. , 2004. A spectrum of biophysical interaction modes between T cells and different antigen-presenting cells during priming in 3-D collagen and in vivo. Blood 104 (9), 2801–2809. [DOI] [PubMed] [Google Scholar]
- Guo X, 2021. Loss of Y chromosome at the interface between aging and Alzheimer’s disease. Cell. Mol. Life Sci 78 (21–22), 7081–7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW, et al. , 2005. Postmenopausal hormone therapy and Alzheimer’s disease risk: interaction with age. J. Neurol. Neurosurg. Psychiatry 76 (1), 103–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepworth MR, Hardman MJ, Grencis RK, 2010. The role of sex hormones in the development of Th2 immunity in a gender-biased model of Trichuris muris infection. Eur. J. Immunol 40 (2), 406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogervorst E, Matthews FE, Brayne C, 2010. Are optimal levels of testosterone associated with better cognitive function in healthy older women and men? Biochim. Biophys. Acta 1800 (10), 1145–1152. [DOI] [PubMed] [Google Scholar]
- Hohman TJ, et al. , 2018. Sex-specific association of apolipoprotein E with cerebrospinal fluid levels of tau. JAMA Neurol. 75 (8), 989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland D, et al. , 2013. Higher rates of decline for women and apolipoprotein E epsilon4 carriers. AJNR Am. J. Neuroradiol 34 (12), 2287–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu B, et al. , 2015. Longitudinal relationships between reproductive hormones and cognitive decline in older men: the concord health and ageing in men project. J. Clin. Endocrinol. Metab 100 (6), 2223–2230. [DOI] [PubMed] [Google Scholar]
- Hua X, et al. , 2010. Sex and age differences in atrophic rates: an ADNI study with n=1368 MRI scans. Neurobiol. Aging 31 (8), 1463–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LK, Chao SP, Hu CJ, 2020. Clinical trials of new drugs for Alzheimer disease. J. Biomed. Sci 27 (1), 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, et al. , 2021. Effects of sex and aging on the immune cell landscape as assessed by single-cell transcriptomic analysis. Proc. Natl. Acad. Sci. U. S. A 118 (33). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson RS, et al. , 2018. The clinical practice of risk reduction for Alzheimer’s disease: a precision medicine approach. Alzheimers Dement. 14 (12), 1663–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson RS, et al. , 2019. Individualized clinical management of patients at risk for Alzheimer’s dementia. Alzheimers Dement. 15 (12), 1588–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C, et al. , 2005. The humoral immune response. Chapter 9. In: Immunobiology. Garland Science, New York. [Google Scholar]
- Jansen WJ, et al. , 2015. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA 313 (19), 1924–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi C, et al. , 2022. CSF-derived CD4(+) T-cell diversity is reduced in patients with Alzheimer clinical syndrome. Neurol. Neuroimmunol. Neuroinflamm 9 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HJ, Shin HS, 2016. Effect of testosterone replacement therapy on cognitive performance and depression in men with testosterone deficiency syndrome. World J. Mens Health 34 (3), 194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahr I, Vandepoele K, van Roy F, 2013. Delta-protocadherins in health and disease. Prog. Mol. Biol. Transl. Sci 116, 169–192. [DOI] [PubMed] [Google Scholar]
- Kanda N, Tamaki K, 1999. Estrogen enhances immunoglobulin production by human PBMCs. J. Allergy Clin. Immunol 103 (2 Pt 1), 282–288. [DOI] [PubMed] [Google Scholar]
- Kanda N, Tsuchida T, Tamaki K, 1996. Testosterone inhibits immunoglobulin production by human peripheral blood mononuclear cells. Clin. Exp. Immunol 106 (2), 410–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, et al. , 2022. Estrogen enhanced the expression of IL-17 by tissue-resident memory gammadeltaT cells from uterus via interferon regulatory factor 4. FASEB J. 36 (2), e22166. [DOI] [PubMed] [Google Scholar]
- Kawas C, et al. , 1997. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology 48 (6), 1517–1521. [DOI] [PubMed] [Google Scholar]
- Kee SJ, et al. , 2012. Age- and gender-related differences in circulating natural killer T cells and their subset levels in healthy Korean adults. Hum. Immunol 73 (10), 1011–1016. [DOI] [PubMed] [Google Scholar]
- Kessel A, et al. , 2012. Human CD19(+)CD25(high) B regulatory cells suppress proliferation of CD4(+) T cells and enhance Foxp3 and CTLA-4 expression in T-regulatory cells. Autoimmun. Rev 11 (9), 670–677. [DOI] [PubMed] [Google Scholar]
- Kim K, et al. , 2021. Therapeutic B-cell depletion reverses progression of Alzheimer’s disease. Nat. Commun 12 (1), 2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissick HT, et al. , 2014. Androgens alter T-cell immunity by inhibiting T-helper 1 differentiation. Proc. Natl. Acad. Sci. U. S. A 111 (27), 9887–9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SL, Flanagan KL, 2016. Sex differences in immune responses. Nat. Rev. Immunol 16 (10), 626–638. [DOI] [PubMed] [Google Scholar]
- Klein SL, Jedlicka A, Pekosz A, 2010. The Xs and Y of immune responses to viral vaccines. Lancet Infect. Dis 10 (5), 338–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf M, et al. , 1993. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature 362 (6417), 245–248. [DOI] [PubMed] [Google Scholar]
- Koran MEI, et al. , 2017. Sex differences in the association between AD biomarkers and cognitive decline. Brain Imag. Behav 11 (1), 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz O, et al. , 2004. Hippocampal synapses depend on hippocampal estrogen synthesis. J. Neurosci 24 (26), 5913–5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronzer VL, Bridges SL Jr., Davis JM 3rd, 2021. Why women have more autoimmune diseases than men: an evolutionary perspective. Evol. Appl 14 (3), 629–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaki T, Kometani K, Ise W, 2015. Memory B cells. Nat. Rev. Immunol 15 (3), 149–159. [DOI] [PubMed] [Google Scholar]
- Larbi A, et al. , 2009. Dramatic shifts in circulating CD4 but not CD8 T cell subsets in mild Alzheimer’s disease. J. Alzheimers Dis 17 (1), 91–103. [DOI] [PubMed] [Google Scholar]
- Lau CF, et al. , 2014. Protective effects of testosterone on presynaptic terminals against oligomeric beta-amyloid peptide in primary culture of hippocampal neurons. Biomed. Res. Int 2014, 103906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc ES, et al. , 2010. Association between sex steroids and cognition in elderly men. Clin. Endocrinol 72 (3), 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Cho HJ, Ryu JH, 2021. Innate immunity and cell death in Alzheimer’s disease. ASN Neuro 13, 17590914211051908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MO, Flavell RA, 2008. TGF-beta: a master of all T cell trades. Cell 134 (3), 392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, et al. , 2017. Age-associated alteration in Th17 cell response is related to endothelial cell senescence and atherosclerotic cerebral infarction. Am. J. Transl. Res 9 (11), 5160–5168. [PMC free article] [PubMed] [Google Scholar]
- Liao Y, et al. , 2022. Ubiquitin specific peptidase 11 as a novel therapeutic target for cancer management. Cell Death Dis. 8 (1), 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin KA, et al. , 2015. Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimers Dement (N Y) 1 (2), 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HY, et al. , 2002. Estrogen inhibition of EAE involves effects on dendritic cell function. J. Neurosci. Res 70 (2), 238–248. [DOI] [PubMed] [Google Scholar]
- Liu Y, et al. , 2018. Cerebrospinal fluid CD4+ T lymphocyte-derived miRNA-let-7b can enhances the diagnostic performance of Alzheimer’s disease biomarkers. Biochem. Biophys. Res. Commun 495 (1), 1144–1150. [DOI] [PubMed] [Google Scholar]
- Liu Y, et al. , 2021. Blockage of IL-17 Ameliorates Aβ-Induced Neurotoxicity and Cognitive Decline.
- Longstreth WT Jr., et al. , 2009. Associations between microinfarcts and other macroscopic vascular findings on neuropathologic examination in 2 databases. Alzheimer Dis. Assoc. Disord 23 (3), 291–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotter H, et al. , 2013. Testosterone increases susceptibility to amebic liver abscess in mice and mediates inhibition of IFNgamma secretion in natural killer T cells. PLoS One 8 (2), e55694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueg G, et al. , 2015. Clinical relevance of specific T-cell activation in the blood and cerebrospinal fluid of patients with mild Alzheimer’s disease. Neurobiol. Aging 36 (1), 81–89. [DOI] [PubMed] [Google Scholar]
- Lundell AC, et al. , 2015. Higher B-cell activating factor levels at birth are positively associated with maternal dairy farm exposure and negatively related to allergy development. J. Allergy Clin. Immunol 136 (4), 1074–1082 e3. [DOI] [PubMed] [Google Scholar]
- Machhi J, et al. , 2021. CD4+ effector T cells accelerate Alzheimer’s disease in mice. J. Neuroinflammation 18 (1), 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield AS, et al. , 2012. Normal ageing is associated with an increase in Th2 cells, MCP-1 (CCL1) and RANTES (CCL5), with differences in sCD40L and PDGF-AA between sexes. Clin. Exp. Immunol 170 (2), 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti PM, Barth JH, 2013. Clinical biochemistry of dihydrotestosterone. Ann. Clin. Biochem 50 (Pt 2), 95–107. [DOI] [PubMed] [Google Scholar]
- Martinez-Cerdeno V, Noctor SC, Kriegstein AR, 2006. Estradiol stimulates progenitor cell division in the ventricular and subventricular zones of the embryonic neocortex. Eur. J. Neurosci 24 (12), 3475–3488. [DOI] [PubMed] [Google Scholar]
- Martinkova J, et al. , 2021. Proportion of women and reporting of outcomes by sex in clinical trials for Alzheimer disease: a systematic review and meta-analysis. JAMA Netw. Open 4 (9), e2124124. [DOI] [PubMed] [Google Scholar]
- Masters CL, et al. , 2015. Alzheimer’s disease. Nat. Rev. Dis. Primers 1, 15056. [DOI] [PubMed] [Google Scholar]
- Masuzawa T, et al. , 1994. Estrogen deficiency stimulates B lymphopoiesis in mouse bone marrow. J. Clin. Invest 94 (3), 1090–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson N, et al. , 2017. Clinical validity of cerebrospinal fluid Abeta42, tau, and phospho-tau as biomarkers for Alzheimer’s disease in the context of a structured 5-phase development framework. Neurobiol. Aging 52, 196–213. [DOI] [PubMed] [Google Scholar]
- McGeer PL, et al. , 1989. Immune system response in Alzheimer’s disease. Can. J. Neurol. Sci 16 (4 Suppl), 516–527. [DOI] [PubMed] [Google Scholar]
- Mendivil-Perez M, et al. , 2019. iPSCs-derived nerve-like cells from familial Alzheimer’s disease PSEN 1 E280A reveal increased amyloid-beta levels and loss of the Y chromosome. Neurosci. Lett 703, 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo S, Sortino MA, 2012. Estrogen activates matrix metalloproteinases-2 and −9 to increase beta amyloid degradation. Mol. Cell. Neurosci 49 (4), 423–429. [DOI] [PubMed] [Google Scholar]
- Miar A, et al. , 2011. Lack of association between protocadherin 11-X/Y (PCDH11X and PCDH11Y) polymorphisms and late onset Alzheimer’s disease. Brain Res. 1383, 252–256. [DOI] [PubMed] [Google Scholar]
- Moffat SD, et al. , 2002. Longitudinal assessment of serum free testosterone concentration predicts memory performance and cognitive status in elderly men. J. Clin. Endocrinol. Metab 87 (11), 5001–5007. [DOI] [PubMed] [Google Scholar]
- Mohamad NV, Soelaiman IN, Chin KY, 2016. A concise review of testosterone and bone health. Clin. Interv. Aging 11, 1317–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molling JW, et al. , 2005. Peripheral blood IFN-gamma-secreting Valpha24+Vbeta11+ NKT cell numbers are decreased in cancer patients independent of tumor type or tumor load. Int. J. Cancer 116 (1), 87–93. [DOI] [PubMed] [Google Scholar]
- Monsonego A, et al. , 2003. Microglia-mediated nitric oxide cytotoxicity of T cells following amyloid beta-peptide presentation to Th1 cells. J. Immunol 171 (5), 2216–2224. [DOI] [PubMed] [Google Scholar]
- Montoya CJ, et al. , 2007. Characterization of human invariant natural killer T subsets in health and disease using a novel invariant natural killer T cell-clonotypic monoclonal antibody, 6B11. Immunology 122 (1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu SC, et al. , 2017. Apolipoprotein E genotype and sex risk factors for Alzheimer disease: a meta-analysis. JAMA Neurol. 74 (10), 1178–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb DC, et al. , 2015. Estrogen and progesterone decrease let-7f microRNA expression and increase IL-23/IL-23 receptor signaling and IL-17A production in patients with severe asthma. J. Allergy Clin. Immunol 136 (4), 1025–34 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistico R, Borg JJ, 2021. Aducanumab for Alzheimer’s disease: a regulatory perspective. Pharmacol. Res 171, 105754. [DOI] [PubMed] [Google Scholar]
- Nord LC, et al. , 2010. Analysis of oestrogen regulation of alpha-, beta- and gamma-secretase gene and protein expression in cultured human neuronal and glial cells. Neurodegener. Dis 7 (6), 349–364. [DOI] [PubMed] [Google Scholar]
- Nutt SL, et al. , 2015. The generation of antibody-secreting plasma cells. Nat. Rev. Immunol 15 (3), 160–171. [DOI] [PubMed] [Google Scholar]
- Oberstein TJ, et al. , 2018. Imbalance of circulating T(h)17 and regulatory T cells in Alzheimer’s disease: a case control study. Front. Immunol 9, 1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paganini-Hill A, Henderson VW, 1996. Estrogen replacement therapy and risk of Alzheimer disease. Arch. Intern. Med 156 (19), 2213–2217. [PubMed] [Google Scholar]
- Pan HF, et al. , 2013. Targeting T-helper 9 cells and interleukin-9 in autoimmune diseases. Cytokine Growth Factor Rev. 24 (6), 515–522. [DOI] [PubMed] [Google Scholar]
- Parachikova A, et al. , 2007. Inflammatory changes parallel the early stages of Alzheimer disease. Neurobiol. Aging 28 (12), 1821–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JC, et al. , 2022. Association of B cell profile and receptor repertoire with the progression of Alzheimer’s disease. Cell Rep. 40 (12), 111391. [DOI] [PubMed] [Google Scholar]
- Pellicanò M, et al. , 2010. Systemic immune responses in Alzheimer’s disease: in vitro mononuclear cell activation and cytokine production. J. Alzheimers Dis 21 (1), 181–192. [DOI] [PubMed] [Google Scholar]
- Plantone D, et al. , 2022. B Lymphocytes in Alzheimer’s disease-a comprehensive review. J. Alzheimers Dis 88 (4), 1241–1262. [DOI] [PubMed] [Google Scholar]
- Polanczyk MJ, et al. , 2004. Cutting edge: estrogen drives expansion of the CD4+CD25 + regulatory T cell compartment. J. Immunol 173 (4), 2227–2230. [DOI] [PubMed] [Google Scholar]
- Pradier C, et al. , 2014. The mini mental state examination at the time of Alzheimer’s disease and related disorders diagnosis, according to age, education, gender and place of residence: a cross-sectional study among the French National Alzheimer database. PLoS One 9 (8), e103630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins ND, Scheltens P, 2015. White matter hyperintensities, cognitive impairment and dementia: an update. Nat. Rev. Neurol 11 (3), 157–165. [DOI] [PubMed] [Google Scholar]
- Quintana-Murci L, Fellous M, 2001. The human Y chromosome: the biological role of a “functional wasteland”. J. Biomed. Biotechnol 1 (1), 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- A.A. R, 2019. Risk factors for Alzheimer’s disease. Folia Neuropathol. 57 (2), 87–105. [DOI] [PubMed] [Google Scholar]
- Ranson T, et al. , 2003. IL-15 availability conditions homeostasis of peripheral natural killer T cells. Proc. Natl. Acad. Sci. U. S. A 100 (5), 2663–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reale M, et al. , 2006. The acetylcholinesterase inhibitor, Donepezil, regulates a Th2 bias in Alzheimer’s disease patients. Neuropharmacology 50 (5), 606–613. [DOI] [PubMed] [Google Scholar]
- Reichardt P, et al. , 2007. Naive B cells generate regulatory T cells in the presence of a mature immunologic synapse. Blood 110 (5), 1519–1529. [DOI] [PubMed] [Google Scholar]
- Resnick SM, et al. , 2017. Testosterone treatment and cognitive function in older men with low testosterone and age-associated memory impairment. JAMA 317 (7), 717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CW, Walker W, Alexander J, 2001. Sex-associated hormones and immunity to protozoan parasites. Clin. Microbiol. Rev 14 (3), 476–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, et al. , 1988. Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer’s disease. Neurobiol. Aging 9 (4), 339–349. [DOI] [PubMed] [Google Scholar]
- Rosario ER, Carroll J, Pike CJ, 2010. Testosterone regulation of Alzheimer-like neuropathology in male 3xTg-AD mice involves both estrogen and androgen pathways. Brain Res. 1359, 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosser EC, Mauri C, 2015. Regulatory B cells: origin, phenotype, and function. Immunity 42 (4), 607–612. [DOI] [PubMed] [Google Scholar]
- Russell LM, et al. , 2007. X chromosome loss and ageing. Cytogenet. Genome Res 116 (3), 181–185. [DOI] [PubMed] [Google Scholar]
- Sachdev PS, et al. , 2009. Sex differences in the causes and consequences of white matter hyperintensities. Neurobiol. Aging 30 (6), 946–956. [DOI] [PubMed] [Google Scholar]
- Saif N, et al. , 2022. Sex-driven differences in the effectiveness of individualized clinical management of Alzheimer’s disease risk. J. Prev. Alzheimers Dis 9 (4), 731–742. [DOI] [PubMed] [Google Scholar]
- Salehi A, Gonzalez Martinez V, Swaab DF, 1998. A sex difference and no effect of ApoE type on the amount of cytoskeletal alterations in the nucleus basalis of Meynert in Alzheimer’s disease. Neurobiol. Aging 19 (6), 505–510. [DOI] [PubMed] [Google Scholar]
- Salem ML, 2004. Estrogen, a double-edged sword: modulation of TH1- and TH2-mediated inflammations by differential regulation of TH1/TH2 cytokine production. Curr. Drug. Targets Inflamm. Allergy 3 (1), 97–104. [DOI] [PubMed] [Google Scholar]
- Sandberg JK, Bhardwaj N, Nixon DF, 2003. Dominant effector memory characteristics, capacity for dynamic adaptive expansion, and sex bias in the innate Valpha24 NKT cell compartment. Eur. J. Immunol 33 (3), 588–596. [DOI] [PubMed] [Google Scholar]
- Saresella M, et al. , 2011. Increased activity of Th-17 and Th-9 lymphocytes and a skewing of the post-thymic differentiation pathway are seen in Alzheimer’s disease. Brain Behav. Immun 25 (3), 539–547. [DOI] [PubMed] [Google Scholar]
- Shao H, et al. , 2012. Hormone therapy and Alzheimer disease dementia: new findings from the Cache County Study. Neurology 79 (18), 1846–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, et al. , 2019. More than one antibody of individual B cells revealed by single-cell immune profiling. Cell Discov. 5, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, et al. , 2022. Impact of anti-amyloid-beta monoclonal antibodies on the pathology and clinical profile of Alzheimer’s disease: a focus on Aducanumab and Lecanemab. Front. Aging Neurosci 14, 870517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomchik MJ, Weisel F, 2012. Germinal center selection and the development of memory B and plasma cells. Immunol. Rev 247 (1), 52–63. [DOI] [PubMed] [Google Scholar]
- Singh RP, Bischoff DS, 2021. Sex hormones and gender influence the expression of markers of regulatory T cells in SLE patients. Front. Immunol 12, 619268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söllvander S, et al. , 2015. Increased number of plasma B cells producing autoantibodies against Abeta42 protofibrils in Alzheimer’s disease. J. Alzheimers Dis 48 (1), 63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, et al. , 2022. Immunotherapy for Alzheimer’s disease: targeting beta-amyloid and beyond. Transl. Neurodegener 11 (1), 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spremo-Potparevic B, et al. , 2004. Analysis of premature centromere division (PCD) of the X chromosome in Alzheimer patients through the cell cycle. Exp. Gerontol 39 (5), 849–854. [DOI] [PubMed] [Google Scholar]
- Spremo-Potparevic B, et al. , 2008. Premature centromere division of the X chromosome in neurons in Alzheimer’s disease. J. Neurochem 106 (5), 2218–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritzer MD, Galea LA, 2007. Testosterone and dihydrotestosterone, but not estradiol, enhance survival of new hippocampal neurons in adult male rats. Dev. Neurobiol 67 (10), 1321–1333. [DOI] [PubMed] [Google Scholar]
- Stowe AM, et al. , 2017. Adaptive lymphocyte profiles correlate to brain Abeta burden in patients with mild cognitive impairment. J. Neuroinflammation 14 (1), 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift-Gallant A, et al. , 2018. Neural androgen receptors affect the number of surviving new neurones in the adult dentate gyrus of male mice. J. Neuroendocrinol 30 (4), e12578. [DOI] [PubMed] [Google Scholar]
- Tai P, et al. , 2008. Induction of regulatory T cells by physiological level estrogen. J. Cell. Physiol 214 (2), 456–464. [DOI] [PubMed] [Google Scholar]
- Tan S, et al. , 2019. Effects of testosterone supplementation on separate cognitive domains in cognitively healthy older men: a meta-analysis of current randomized clinical trials. Am. J. Geriatr. Psychiatry 27 (11), 1232–1246. [DOI] [PubMed] [Google Scholar]
- Tang MX, et al. , 1996. Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease. Lancet 348 (9025), 429–432. [DOI] [PubMed] [Google Scholar]
- Teixeira D, et al. , 2011. Evaluation of lymphocyte levels in a random sample of 218 elderly individuals from Sao Paulo city. Rev. Bras. Hematol. Hemoter 33 (5), 367–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tifratene K, et al. , 2015. Progression of mild cognitive impairment to dementia due to AD in clinical settings. Neurology 85 (4), 331–338. [DOI] [PubMed] [Google Scholar]
- Togo T, et al. , 2002. Occurrence of T cells in the brain of Alzheimer’s disease and other neurological diseases. J. Neuroimmunol 124 (1–2), 83–92. [DOI] [PubMed] [Google Scholar]
- Town T, et al. , 2002. Reduced Th1 and enhanced Th2 immunity after immunization with Alzheimer’s beta-amyloid(1–42). J. Neuroimmunol 132 (1–2), 49–59. [DOI] [PubMed] [Google Scholar]
- Tsiknia AA, et al. , 2022. Sex differences in plasma p-tau181 associations with Alzheimer’s disease biomarkers, cognitive decline, and clinical progression. Mol. Psychiatry 27 (10), 4314–4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RS, et al. , 2020. Potential new approaches for diagnosis of Alzheimer’s disease and related dementias. Front. Neurol 11, 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaananen HK, Harkonen PL, 1996. Estrogen and bone metabolism. Maturitas 23 (Suppl), S65–S69. [DOI] [PubMed] [Google Scholar]
- van den Heuvel DM, et al. , 2004. Different progression rates for deep white matter hyperintensities in elderly men and women. Neurology 63 (9), 1699–1701. [DOI] [PubMed] [Google Scholar]
- van Dyck CH, et al. , 2023. Lecanemab in early Alzheimer’s disease. N. Engl. J. Med 388 (1), 9–21. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, et al. , 2008. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat. Immunol 9 (12), 1341–1346. [DOI] [PubMed] [Google Scholar]
- Vermeulen MC, et al. , 2022. Mosaic loss of Chromosome Y in aged human microglia. Genome Res. 32 (10), 1795–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignali DA, Collison LW, Workman CJ, 2008. How regulatory T cells work. Nat. Rev. Immunol 8 (7), 523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahjoepramono EJ, et al. , 2016. The effects of testosterone supplementation on cognitive functioning in older men. CNS Neurol. Disord. Drug Targets 15 (3), 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walecki M, et al. , 2015. Androgen receptor modulates Foxp3 expression in CD4+CD25 +Foxp3+ regulatory T-cells. Mol. Biol. Cell 26 (15), 2845–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker KA, Ficek BN, Westbrook R, 2019. Understanding the role of systemic inflammation in Alzheimer’s disease. ACS Chem. Neurosci 10 (8), 3340–3342. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang M, Liu H, 2019. LncRNA17A regulates autophagy and apoptosis of SH-SY5Y cell line as an in vitro model for Alzheimer’s disease. Biosci. Biotechnol. Biochem 83 (4), 609–621. [DOI] [PubMed] [Google Scholar]
- Waters A, N. Society for Women’s Health Research Alzheimer’s Disease, Laitner MH, 2021. Biological sex differences in Alzheimer’s preclinical research: A call to action. Alzheimers Dement (N Y) 7 (1) e12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton W, et al. , 2019. Interleukin 9 alterations linked to alzheimer disease in African Americans. Ann. Neurol 86 (3), 407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer RA, et al. , 2011. Timing of hormone therapy and dementia: the critical window theory revisited. Ann. Neurol 69 (1), 163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, et al. , 1990. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J. Neurosci 10 (12), 4035–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao ZM, et al. , 2009. Estrogen regulation of the neprilysin gene through a hormone-responsive element. J. Mol. Neurosci 39 (1–2), 22–26. [DOI] [PubMed] [Google Scholar]
- Yan Y, et al. , 2022. X-linked ubiquitin-specific peptidase 11 increases tauopathy vulnerability in women. Cell 185 (21), 3913–3930 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M, et al. , 2008. Androgens regulate neprilysin expression: role in reducing beta-amyloid levels. J. Neurochem 105 (6), 2477–2488. [DOI] [PubMed] [Google Scholar]
- Yao PL, et al. , 2017. Androgen alleviates neurotoxicity of beta-amyloid peptide (Abeta) by promoting microglial clearance of Abeta and inhibiting microglial inflammatory response to Abeta. CNS Neurosci. Ther 23 (11), 855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M, et al. , 2022. Androgens regulate tau phosphorylation through phosphatidylinositol 3-kinase-protein kinase B-glycogen synthase kinase 3beta signaling. Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Z, et al. , 2022. KDM6A regulates cell plasticity and pancreatic cancer progression by noncanonical activin pathway. Cell Mol. Gastroenterol. Hepatol 13 (2), 643–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F, et al. , 2015. The perimenopausal aging transition in the female rat brain: decline in bioenergetic systems and synaptic plasticity. Neurobiol. Aging 36 (7), 2282–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y, et al. , 2022. Sex differences in the progression of metabolic risk factors in diabetes development. JAMA Netw. Open 5 (7), e2222070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurov YB, et al. , 2014. X chromosome aneuploidy in the Alzheimer’s disease brain. Mol. Cytogenet 7 (1), 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandi PP, et al. , 2002. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA 288 (17), 2123–2129. [DOI] [PubMed] [Google Scholar]
- Zeng J, et al. , 2022. JKAP, Th1 cells, and Th17 cells are dysregulated and intercorrelated, among them JKAP and Th17 cells relate to cognitive impairment progression in Alzheimer’s disease patients. Ir. J. Med. Sci 191 (4), 1855–1861. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Niu C, 2022. Relation of CDC42, Th1, Th2, and Th17 cells with cognitive function decline in Alzheimer’s disease. Ann. Clin. Transl. Neurol 9 (9), 1428–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Simpkins JW, 2010. Okadaic acid induces tau phosphorylation in SH-SY5Y cells in an estrogen-preventable manner. Brain Res. 1345, 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, et al. , 2004. Estrogen and androgen protection of human neurons against intracellular amyloid beta1–42 toxicity through heat shock protein 70. J. Neurosci 24 (23), 5315–5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, et al. , 2013. Th17 cell-mediated neuroinflammation is involved in neurodegeneration of abeta1–42-induced Alzheimer’s disease model rats. PLoS One 8 (10), e75786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang FJ, et al. , 2018. Prevalence of white matter hyperintensities increases with age. Neural Regen. Res 13 (12), 2141–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumoff B, et al. , 1995. Twenty-four-hour mean plasma testosterone concentration declines with age in normal premenopausal women. J. Clin. Endocrinol. Metab 80 (4), 1429–1430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.


