Abstract
Background:
The global coronavirus disease-19 (COVID-19) pandemic has resulted in procedural delays around the world; however, timely and aggressive surgical resection for malignant brain tumor patients is essential for outcome optimization. To investigate the association between COVID-19 and outcomes of these patients, we queried the 2020 National Inpatient Sample (NIS) for differences in rates of surgical resection, time to surgery, mortality, and discharge disposition between patients with and without confirmed COVID-19 infection.
Methods:
Patient data were taken from the NIS from April 2020 to December 2020. COVID-19 diagnosis was determined with the International Classification of Diseases, Tenth Revision, Clinical Modification code U07.1.
Results:
A total of 30,671 malignant brain tumor patients met inclusion criteria and 738 (2.4%) patients had a confirmed COVID-19 diagnosis. COVID-19-positive patients had lower likelihood of receiving surgery (Odds ratio [OR] 0.43, 95% confidence interval [CI] 0.29–0.63, P < 0.0001), increased likelihood of mortality (OR 2.18, 95% CI 1.78–2.66, P < 0.0001), and increased likelihood of non-routine discharge (OR 1.25, 95% CI 1.13–1.39, P < 0.0001). Notably, COVID patients receiving surgery were not associated with surgical delay (P = 0.17).
Conclusion:
COVID-19 infection was associated with worse patient outcome in malignant brain tumor patients, including decreased likelihood of receiving surgery, increased likelihood of mortality, and increased likelihood of non-routine discharge. Our study highlights the need to balance the risks and benefits of delaying surgery for malignant brain tumor patients with COVID-19. Although the COVID-19 pandemic is no longer a public health emergency, understanding the pandemic’s impact on outcome provides important insight in effective triage for these patients in the situations where resources are limited.
Keywords: Brain tumor, Coronavirus, Coronavirus disease-19, Malignant, National inpatient sample

INTRODUCTION
SARS-CoV-2, the virus that causes coronavirus disease-19 (COVID-19), was first identified in December 2019 and by March 2020, it was declared a global pandemic.[36] COVID-19 strained the health-care system resulting in the triaging of patients and rationing of supplies.[2] In response, a series of rapidly evolving guidelines for healthcare were created to minimize the spread of COVID-19, ensure patient safety, and divert resources to the care of COVID-19 patients. As a result, the management of many chronic diseases, including cancer, was drastically altered.[1,11] Hospitals reduced or stopped elective surgeries following the guidance of state and federal agencies and medical associations.[21,22] In this instance, the definition of “elective” included a number of cancer cases and, as such, many neurosurgical departments suffered delays or cancellations in malignant tumor resections.[5,12] By the end of March 2020, an estimated 18–44.8% of all cancer surgeries were canceled in the United States.[7] In turn, this led to the American College of Surgeons and the American Association of Neurological Surgeons classifying malignant brain tumors as requiring emergent intervention due the poor prognosis of the tumors and worse outcomes if cancer surgeries were delayed by 3 months.[8,26,32] Still, neurosurgeons had to balance the benefits of emergently operating on COVID-19-positive patients with malignant tumors and the unknown risk of COVID-19 on outcomes.
Approximately 25,000 cases of malignant central nervous system cancers occur annually in the United States, representing only 1.4% of total cancer incidence.[30,31] Metastases are the most common malignant brain tumor while the most common primary malignant brain tumor is glioblastoma, which accounts for 14.2% of all brain tumors. Malignant brain tumors as a whole have a very poor prognosis (35.7% 5-year relative survival rate).[23] The current standard of practice to improve patient survival is surgical resection of high-grade brain tumors with subsequent chemoradiation.[20] Timely and aggressive surgical resection of malignant brain tumor increases overall survival in patients.[13,18,27] Resection of the tumor not only reduces the tumor burden but also relieves the mass effect on the brain to preserve neurological function.[35] However, resection is not curative as malignant brain tumors have high reoccurrence rates.[37] As such, given both the poor prognosis and rapid expansion of such tumors, prompt surgical intervention is vital to improving patient outcomes and survival.
For patients with malignant brain tumors who are COVID-19-positive, the risks and benefits of urgent resection must be weighed. The American Society of Anesthesiologists guidelines recommend waiting 7 weeks after COVID-19 diagnosis for surgeries when possible to minimize postoperative risks.[3] However, as malignant tumors are often considered emergent, waiting 7 weeks is not always an option. Notably, we have demonstrated in national data that COVID-19 diagnosis has been associated with delays in benign brain tumor surgery. However, the effect of COVID diagnosis on malignant tumors with the described more urgent indications remains unclear. Further, the full risks associated with tumor resection for COVID-19 patients are not known due to limited research and data; prior limited studies have shown an association between COVID-19 and malignant brain tumors with poorer outcomes but are limited by small sample size.[16] As the pandemic transitions into an endemic phase, it is vital to understand the effect of COVID-19 on postoperative outcomes to maximize treatment efficiency and patient survival, and to optimize further triage management of these patients in future public health emergencies.
In this study, we utilize the National Inpatient Sample (NIS) database to investigate the national rates of surgical resection, mortality, and unfavorable discharge in COVID-19-positive patients with malignant brain tumors. To the best of our knowledge, our study is the first to use national data to analyze the association of a COVID-19 diagnosis with the outcomes of likelihood of receiving surgery, mortality, and discharge disposition in malignant brain tumor patients.
MATERIALS AND METHODS
Data source
The Healthcare Cost and Utilization Project (HCUP), an initiative by the Agency for Healthcare Research and Quality (AHRQ), releases the NIS annually. This comprehensive database is the most extensive publicly available inpatient health-care database in the United States and covers over 97% of the national population. The NIS comprises approximately 7 million hospital stays each year without weighting, and about 35 million hospitalizations with weighting.[19]
Study population
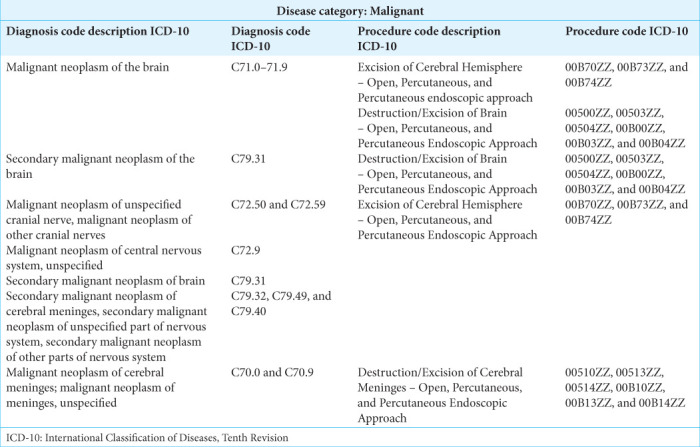
This study is a retrospective database analysis of NIS data from April 2020 to December 2020, which are the first dates to include the International Classification of Diseases, Tenth Revision (ICD-10) diagnosis code (U07.1) for COVID-19 infection. Patients diagnosed with malignant neoplasms of the brain were included in the study. Patients with benign brain tumors were excluded from the cohort. A full list of ICD-10 codes used in inclusion/exclusion criteria can be found in Supplementary Table S1. Patients who were under 18 years old, missing in-hospital survival data, missing length of stay data, or missing discharge disposition were also excluded from the study. Figure 1 provides a breakdown of the patient selection process.
Figure 1:
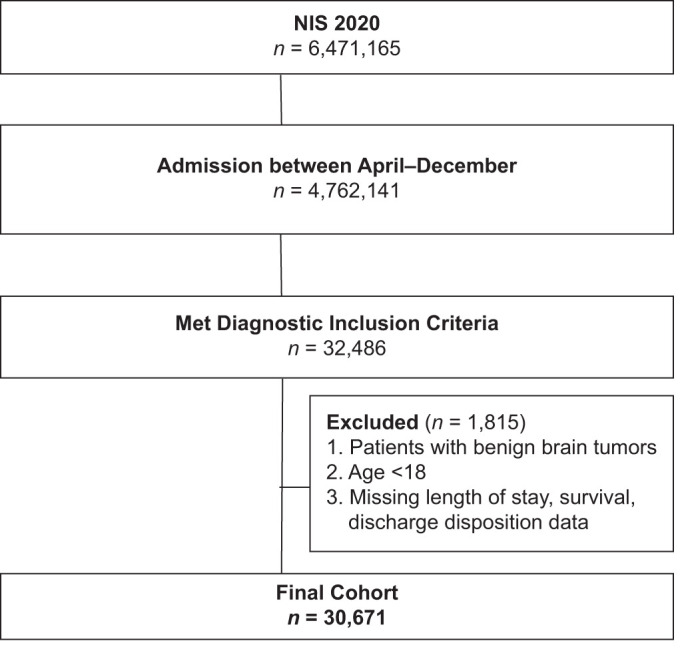
Flowchart detailing the inclusion and exclusion process in determining the final study cohort. n = Number, NIS = National Inpatient Sample.
Study variables
To conduct univariate and multivariate analyses, data were extracted from the NIS database for patient, hospital, and clinical information. COVID-19 was the primary exposure variable (ICD-10 code U07.1). Patient characteristics analyzed included age, sex, race, insurance status/payer, All
Patient Refined Diagnosis Related Groups (APR-DRG) Risk of, APR-DRG Severity of illness, Elixhauser comorbidity index (ECI), patient residential location, and income. Hospital characteristics include bed size (i.e., number of hospital beds), control/ownership of hospital, census division of the hospital, and census region of hospital. Outcome variables included surgical resection (on a binary yes/no scale), in-hospital mortality, discharge disposition (unfavorable vs. favorable), and transfer status. A full breakdown of each variable used in the study as shown in Table 1. Patients who underwent surgical resection were also analyzed for the amount of time from admission to surgery.
Table 1:
General patient demographics of all malignant brain tumor patients that met inclusion and exclusion criteria, stratified by COVID-19 diagnosis.
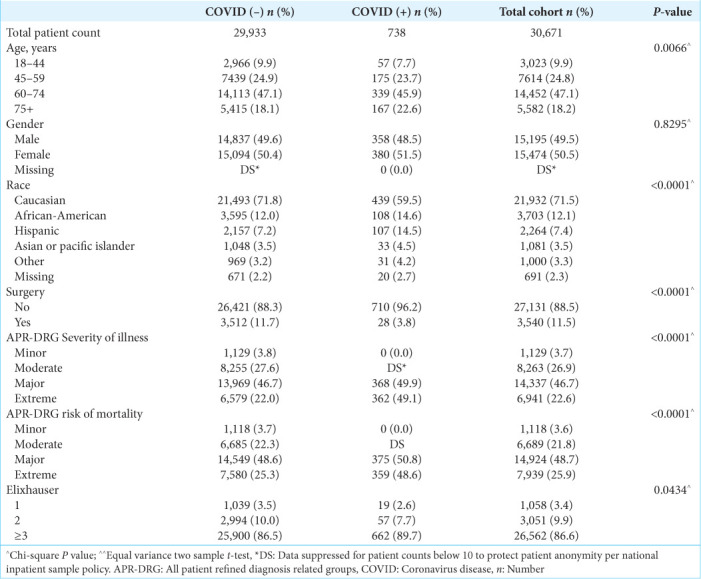
The above covariates were utilized to evaluate the effect of COVID-19 on the primary outcomes of (1) rate of surgical resection, (2) in-hospital mortality, and (3) discharge disposition. APR-DRG risk of mortality and disease severity was assigned using software developed by 3M Health Information Systems, and the ECI was grouped into four categories (0, 1, 2, and ≥3 factors) based on an ICD-10 coding algorithm.
Statistical analysis
Descriptive analyses of patient demographics and clinical characteristics were stratified by COVID-19 status using mean (standard deviation) and t-test for continuous variables and frequency (percentage) and χ2 tests for categorical variables. Two types of multivariable regression were then utilized to analyze outcomes: (1) logistic regression for surgical resection and in-hospital mortality and (2) log-binomial regression for discharge disposition and transfer-in status. Generalized estimation equations were used in all models to account for hospital clustering. The logistic model assumptions were checked using the Hosmer-Lemeshow goodness-of-fit test. All of the covariates mentioned earlier were included in the model for adjustment. Age was recategorized as it violated the linearity assumption. Only complete cases were used for regressions without imputation since missing data affected <4% of records. P < 0.05 was considered statistically significant. The analysis was performed using SAS, version 9.4 (SAS Institute, Cary, North Carolina, USA).
RESULTS
Patient demographics
A total of 30,671 patients met inclusion and exclusion criteria. Within this group, 738 (2.4%) patients had a COVID-19 diagnosis. A flowchart outlining the inclusion and exclusion process is shown in Figure 1. General characteristics of COVID-19-positive patients include older age (age 75+, 22.6% vs. 18.1%), higher APR-DRG risk of mortality (extreme, 48.6% vs. 25.3%), and severity of illness (extreme, 49.1% vs. 22.0%) [Table 1]. Additional patient characteristics pertaining to hospital stay are shown in Table 2.
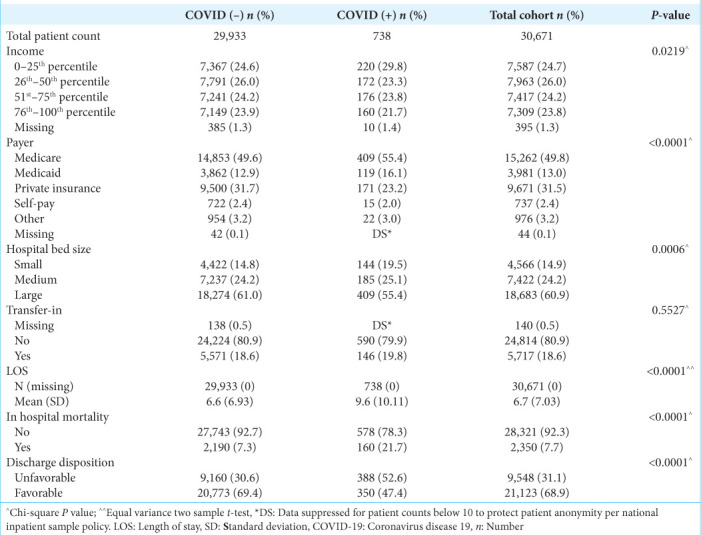
Table 2:
Additional patient demographics pertaining to hospital stay of all malignant brain tumor patients that met inclusion and exclusion criteria, stratified by COVID-19 diagnosis.
Surgical resection cohort
COVID-19 patients who underwent surgical resection for malignant brain tumors trended toward a longer time from admission to surgery (3.1 days for COVID-19 patients vs. 2.1 days for patients without COVID-19); however, this finding was not statistically significant (P = 0.17). More COVID-19 patients also had 3 or more days from admission to surgery compared to patients without COVID-19 infection (≥3, 42.9% vs. 39.0%), but this difference was also not statistically significant (P = 0.25).
Patient outcomes
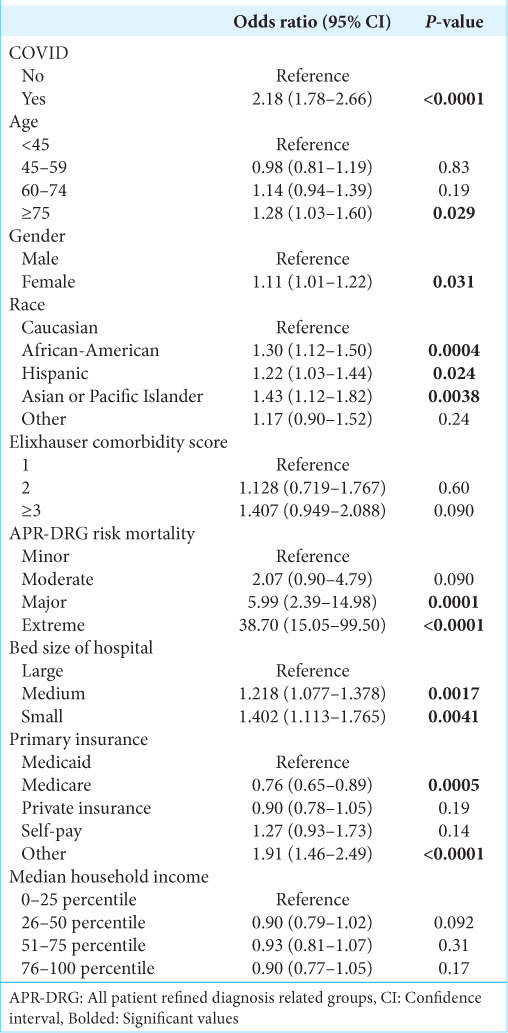
After controlling for other covariates, COVID-19 infection was significantly associated with a decreased likelihood of receiving surgery (Odds ratio [OR] 0.43, 95% confidence interval [CI] 0.29–0.63, P < 0.0001) [Table 2], an increased likelihood of mortality (OR 2.18, 95% CI 1.78–2.66, P < 0.0001) [Table 3], and increased likelihood of non-routine discharge (OR 1.25, 95% CI 1.13–1.39, P < 0.0001) [Table 4].
Table 3:
Factors associated with likelihood of receiving surgical resection for malignant brain tumor patients.
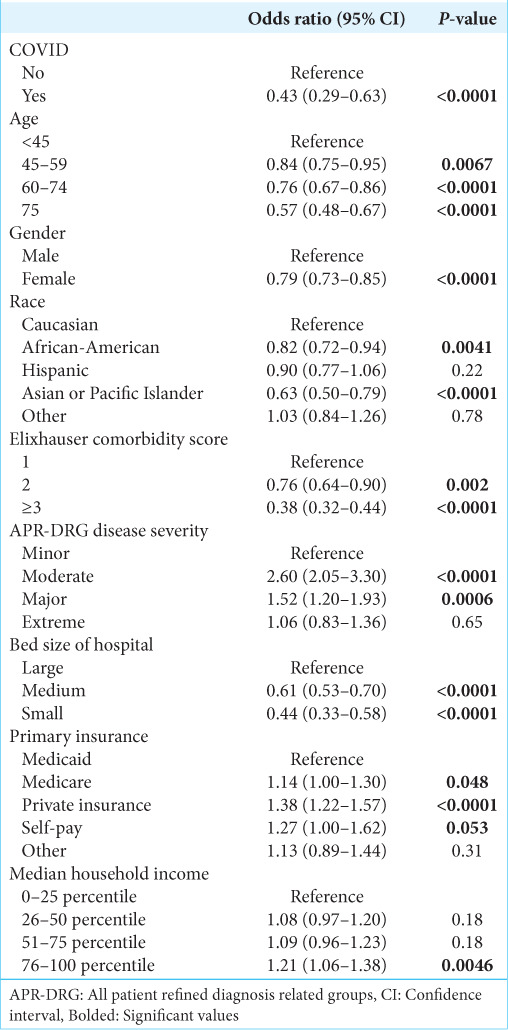
Table 4:
Factors associated with likelihood of in-hospital mortality for malignant brain tumor patients.
Sub-cohort analysis of malignant primary tumors and malignant secondary (metastatic) tumors demonstrated similar results. In patients with malignant primary tumors, COVID-19 infection was associated with a decreased likelihood of receiving surgery (OR 0.37, 95% CI 0.22–0.62, P < 0.0002), increased likelihood of mortality (OR 1.91, 95% CI 1.15–3.16, P = 0.01), and increased likelihood of non-routine discharge (OR 1.19, 95% CI 1.01–1.40, P = 0.04). In patients with malignant secondary tumors, COVID-19 infection was associated with a decreased likelihood of receiving surgery (OR 0.37, 95% CI 0.20–0.68, P = 0.001), increased likelihood of mortality (OR 2.36, 95% CI 1.88– 2.96, P < 0.0001), and increased likelihood of non-routine discharge (OR 1.24, 95% CI 1.08–1.43, P = 0.002).
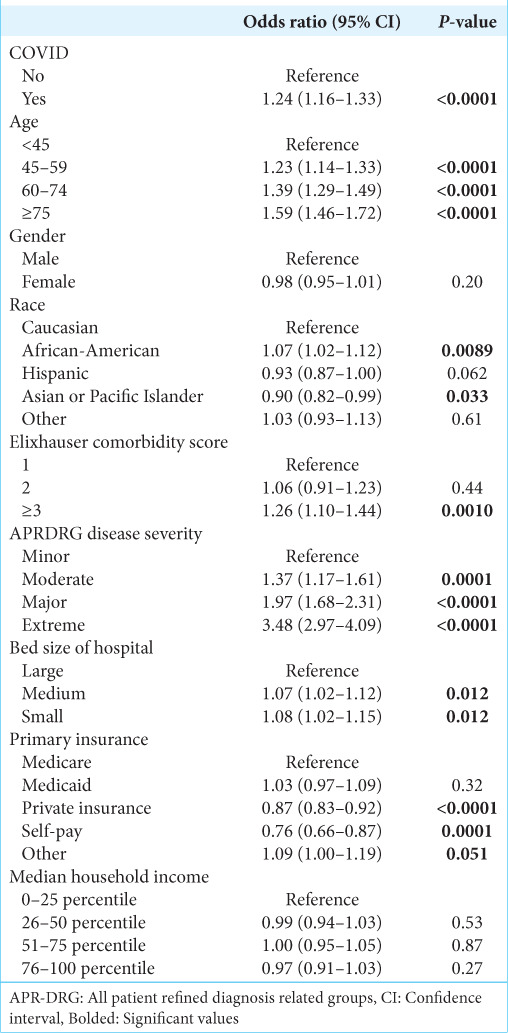
Additional characteristics associated with likelihood of receiving surgery are shown in Table 3. The characteristics associated with a decreased likelihood of receiving surgery were older age (60–74-years-old: OR 0.76, 95% CI 0.67–0.86, P < 0.0001; 75+ years old: OR 0.57, 95% CI 0.48–0.67, P < 0.0001) and ECI ≥3 (OR 0.38, 95% CI 0.32–0.44, P < 0.0001). Factors associated with increased likelihood of receiving surgery included private insurance (OR 1.38, 95% CI 1.22–1.57, P < 0.0001) and median income in the highest quartile (OR 1.21, 95% CI 1.06–1.38, P < 0.0001).
The factors associated with increased likelihood of mortality are shown in Table 4. Other than COVID-19 infection, characteristics associated with increased likelihood of mortality include older age (75+ years old: OR 1.28, 95% CI 1.03–1.60, P < 0.03) and non-white race.
Factors associated with likelihood of non-routine discharge are shown in Table 5. These included higher APR-DRG disease severity, ECI ≥3 (OR 1.25, 95% CI 1.07–1.47, P = 0.005), and age over 45 years old.
Table 5:
Factors associated with likelihood of unfavorable discharge for malignant brain tumor patients.
DISCUSSION
In our analysis of the NIS, malignant brain tumor patients with COVID-19 were associated with a decreased likelihood of receiving surgery, increased likelihood of in-hospital mortality, and increased likelihood of non-routine discharge. Notably, despite a decreased likelihood of surgery among COVID patients, those COVID patients receiving surgery did not demonstrate an association with surgical delay. To date, this study is the first to identify these findings using a large, national dataset and the findings of our study provide important insight into optimal management of these patients in resource-scarce circumstances like the COVID-19 pandemic.
Surgical resection is an essential component in the treatment of many malignant brain tumors. However, during the COVID-19 pandemic, many hospitals had to postpone or cancel non-emergent surgeries due to concerns about the risk of infection and need to preserve hospital resources. While often necessary, malignant tumor resections are typically not “emergent” and, as such, were often classified as “elective.” However, due to the aggressive nature of malignant brain tumors, delaying surgery up to 7 weeks – as prior guidelines have recommended – is not always feasible.[17] Fortunately, we did note that COVID diagnosis was not necessarily associated with delay in surgery when patients were offered surgical resection. Further, prior limited studies have shown an association between COVID-19 and malignant brain tumors with poorer outcomes.[16] One study from the UK with 1221 patients found that the pandemic changed malignant brain tumor management, most commonly resulting in patients receiving no surgery or no treatment at all.[24] However, this study is limited by small sample size and limited generalizability and does not compare outcome between COVID-19-positive patients and COVID-19-negative patients. Nevertheless, our study with a large, national dataset reinforces the findings from this prior study with a larger sample size.
In addition, our study found that patients with concurrent COVID-19 infection had 2.18 times greater odds of mortality than those without COVID-19. Patients with both cancer and COVID-19 infection pose a unique clinical dilemma – in the interest of not advancing their COVID-19 infection, it may be sensible to withhold chemotherapy and steroid treatment so as to not further immunosuppress these patients.[34] In addition, COVID-19 treatments themselves may immunosuppress patients allowing for metastatic progression.[29] The largest study to date of 869 neurosurgical and neurointerventional patients at two medical centers demonstrated that those with a concurrent COVID-19 diagnosis are associated with an increased risk of mortality (18.2% vs. 4.1%, P = 0.0029).[28] The same study also found COVID-19 to be associated with non-routine discharge, which was similarly identified in our study. One international meta-analysis found that the pooled mortality risk estimate for hospitalized patients with COVID-19 and cancer was 14.1%, at least 5 times greater than the mortality risk of non-elderly patients with COVID-19.[38] Furthermore, a small study of patients with primary brain tumors and COVID-19 in the Netherlands found that half of the patients had a severe disease course requiring hospital admission and 13% had a fatal outcome.[9] A recent cohort study in China found that patients with brain cancer are at increased risk for COVID-19 mortality.[6]
Further analysis is necessary to elucidate the etiology of increased mortality among patients with brain tumors who have COVID-19. Intrinsic medical factors, such as cytokine storm, neuroinflammation, and endothelial cell destruction secondary to COVID-19 infection, could contribute to worse outcomes in brain tumor patients.[10,25] Other logistical contributors to worse outcomes could be delays in cancer treatment, immunosuppression from chemotherapy, and limited hospital resources.[12]
Our analysis also found demographic differences between patients with malignant brain tumors with and without COVID-19. A higher percentage of malignant brain tumor patients with COVID-19 were African-American (14.9%) and Hispanic (14.5%) compared to patients without COVID-19 (12% were African-American and 7.2% were Hispanic). Racial disparities in COVID-19 infections and outcomes have been well documented in the United States; African-American and Hispanic populations have an estimated 1.5–3.5 higher risk and 1.3–7.7 higher risk, respectively, compared to white populations.[14,15,33] Our data further validate these trends among malignant brain tumor patients in the United States. In addition, we found that malignant brain tumor patients with COVID-19 were significantly more likely to have Medicare or Medicaid rather than private insurance compared to malignant brain tumor patients without COVID-19. Insurance status has been linked with hospitalization rates from COVID-19; one 2020 retrospective and cohort study found that patients with Medicaid were more than 2 times as likely to admitted to a hospital for COVID-19 than those with commercial insurance.[4] One retrospective and cohort study conducted in Georgia found that what was assumed to be a racial disparity in COVID-19 mortality can be attributed to insurance status; after controlling for insurance status, African-American and Caucasian patients had similar mortality outcomes, but patients with private insurance had significantly lower mortality rates than those with Medicare (OR = 0.68, 95% CI 0.48–0.96).[17] Further analysis is needed to determine the impact of race and insurance status on outcomes among patients with malignant brain tumors with and without COVID-19.
Limitations
Our retrospective and cohort study, which utilized the NIS, has several inherent limitations. Although the NIS contains a large number of patients, only 1 year of 2020 data has been made public so far, limiting the number of documented COVID-19 patients who underwent tumor resection. The analysis was therefore underpowered, which may affect the statistical significance noted in assessing the association of COVID with time to surgery. The NIS also cannot identify patients with multiple hospitalizations, resulting in readmission being recorded as new patients. This limits the ability to track patients with long-term conditions and assess for long-term cancer survival. Furthermore, the NIS does not distinguish pre-existing conditions and hospital-acquired conditions. In addition, the NIS is specific to the United States, and since the impact of the COVID-19 pandemic varies greatly across different regions and countries, NIS data may not be generalizable. Finally, as with any administrative database, reliance on ICD-10 coding can result in errors and subjectivity in data input and coding. Despite these limitations, our study provides valuable insights into the relationship between malignant brain tumors and COVID-19. Further research is needed to validate our findings and to address the limitations of the NIS.
CONCLUSION
Overall, malignant brain tumor patients with COVID-19 infection were associated with worse overall outcomes, including decreased likelihood of receiving surgery, increased likelihood of mortality, and increased likelihood of non-routine discharge. To the best of our knowledge, this study is the first to analyze the relationship of these outcomes with COVID-19 infection with a large national dataset. The findings of our study emphasize the need to balance the risks and benefits of delaying surgery for malignant brain tumor patients with COVID-19. Further study on causal relationships between COVID-19 and patient outcomes is needed to optimize management for this patient population at risk for direct effects of COVID infection and indirect health-care system consequences of COVID-19. As such, though the COVID-19 pandemic is no longer a public health emergency, reinforcing our understanding of the pandemic’s impact on outcome provides important insight in effective triage for these patients in future situations where resources are limited.
Acknowledgments
Li Ding was supported by grants UL1TR001855 from the National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
How to cite this article: Liu KQ, Dallas J, Wenger TA, Richards H, Ding L, Chow FE, et al. Coronavirus disease-19 is associated with decreased treatment access and worsened outcomes in malignant brain tumor patients. Surg Neurol Int 2023;14:292.
Contributor Information
Kristie Qwan-Ting Liu, Email: kqliu@usc.edu.
Jonathan Dallas, Email: jonathan.dallas@med.usc.edu.
Talia A. Wenger, Email: tawenger@usc.edu.
Hunter Richards, Email: hunter.g.richards@kp.org.
Li Ding, Email: li.ding@med.usc.edu.
Frances Elaine Chow, Email: frances.chow@med.usc.edu.
Gabriel Zada, Email: gzada@usc.edu.
William J. Mack, Email: william.mack@med.usc.edu.
Frank J. Attenello, Email: frank.attenello@med.usc.edu.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Li Ding was supported by grants UL1TR001855 from the National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
SUPPLEMENTARY TABLE
Supplementary Table S1:
ICD-10 Codes used in inclusion criteria.
REFERENCES
- 1.Al-Shamsi HO, Alhazzani W, Alhuraiji A, Coomes EA, Chemaly RF, Almuhanna M, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: An international collaborative group. Oncologist. 2020;25:e936–45. doi: 10.1634/theoncologist.2020-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anesi GL, Lynch Y, Evans L. A conceptual and adaptable approach to hospital preparedness for acute surge events due to emerging infectious diseases. Crit Care Explor. 2020;2:e0110. doi: 10.1097/CCE.0000000000000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ASA and APSF Joint Statement on Elective Surgery/Procedures and Anesthesia for Patients after COVID-19 Infection. 2022. Available from: https://www.apsf.org/wp-content/uploads/news-updates/2022/ASA-APSF-Joint-Statement-Elective-Surgery-2022-02-22.pdf [Last accessed on 2023 Mar 07]
- 4.Azar KM, Shen Z, Romanelli RJ, Lockhart SH, Smits K, Robinson S, et al. Disparities in outcomes among COVID-19 patients in a large health care system in California. Health Aff (Millwood) 2020;39:1253–62. doi: 10.1377/hlthaff.2020.00598. [DOI] [PubMed] [Google Scholar]
- 5.Burke JF, Chan AK, Mummaneni V, Chou D, Lobo EP, Berger MS, et al. Letter: The coronavirus disease 2019 global pandemic: A neurosurgical treatment algorithm. Neurosurgery. 2020;87:E50–6. doi: 10.1093/neuros/nyaa116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chai C, Feng X, Lu M, Li S, Chen K, Wang H, et al. One-year mortality and consequences of COVID-19 in cancer patients: A cohort study. IUBMB Life. 2021;73:1244–56. doi: 10.1002/iub.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collaborative CO. Elective surgery cancellations due to the COVID-19 pandemic: Global predictive modelling to inform surgical recovery plans. Br J Surg. 2020;107:1440–9. doi: 10.1002/bjs.11746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.COVID-19: Guidance for Triage of Non-Emergent Surgical Procedures. 2020. Available from: https://www.facs.org/formedical-professionals/covid-19/clinical-guidance/triage [Last accessed on 2023 Mar 07]
- 9.de Joode K, Taal W, Snijders TJ, Hanse M, Koekkoek JA, Oomen-de Hoop E, et al. Patients with primary brain tumors and COVID-19: A report from the Dutch Oncology COVID-19 Consortium. Neuro Oncol. 2022;24:326–8. doi: 10.1093/neuonc/noab258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kempuraj D, Selvakumar GP, Ahmed ME, Raikwar SP, Thangavel R, Khan A, et al. COVID-19, mast cells, cytokine storm, psychological stress, and neuroinflammation. Neuroscientist. 2020;26:402–14. doi: 10.1177/1073858420941476. [DOI] [PubMed] [Google Scholar]
- 11.Kendzerska T, Zhu DT, Gershon AS, Edwards JD, Peixoto C, Robillard R, et al. The effects of the health system response to the COVID-19 pandemic on chronic disease management: A narrative review. Risk Manag Healthc Policy. 2021;14:575–84. doi: 10.2147/RMHP.S293471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kessler RA, Zimering J, Gilligan J, Rothrock R, McNeill I, Shrivastava RK, et al. Neurosurgical management of brain and spine tumors in the COVID-19 era: An institutional experience from the epicenter of the pandemic. J Neurooncol. 2020;148:211–9. doi: 10.1007/s11060-020-03523-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo C, Song K, Wu S, Hameed NU, Kudulaiti N, Xu H, et al. The prognosis of glioblastoma: A large, multifactorial study. Br J Neurosurg. 2021;35:555–61. doi: 10.1080/02688697.2021.1907306. [DOI] [PubMed] [Google Scholar]
- 14.Mackey K, Ayers CK, Kondo KK, Saha S, Advani SM, Young S, et al. Racial and ethnic disparities in COVID-19-related infections, hospitalizations, and deaths: A systematic review. Ann Intern Med. 2021;174:362–73. doi: 10.7326/M20-6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magesh S, John D, Li WT, Li Y, Mattingly-App A, Jain S, et al. Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status: A systematic-review and meta-analysis. JAMA Netw Open. 2021;4:e2134147. doi: 10.1001/jamanetworkopen.2021.34147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marenco-Hillembrand L, Erben Y, Suarez-Meade P, Franco-Mesa C, Sherman W, Eidelman BH, et al. Outcomes and surgical considerations for neurosurgical patients hospitalized with COVID-19-A multicenter case series. World Neurosurg. 2021;154:e118–29. doi: 10.1016/j.wneu.2021.06.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCain JL, Wang X, Connell K, Morgan J. Assessing the impact of insurance type on COVID-19 mortality in black and white patients in the largest healthcare system in the state of georgia. J Natl Med Assoc. 2022;114:218–26. doi: 10.1016/j.jnma.2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molinaro AM, Hervey-Jumper S, Morshed RA, Young J, Han SJ, Chunduru P, et al. Association of maximal extent of resection of contrast-enhanced and non-contrast-enhanced tumor with survival within molecular subgroups of patients with newly diagnosed glioblastoma. JAMA Oncol. 2020;6:495–503. doi: 10.1001/jamaoncol.2019.6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National (Nationwide) Inpatient Sample (NIS) Database Documentation. Available from: https://hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp [Last accessed on 2023 Mar 05]
- 20.NCCN Guidelines Version 2.2022. 2022 Available from: https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf [Last accessed on 2023 Mar 07] [Google Scholar]
- 21.Newson G, Gilbert BP. United States: Department of Health Care Services, State of California Health and Human Services Agency; 2020. Medi-Cal Guidance Relating to Non-Urgent, Non-Essential, or Elective Procedures Relative to the 2019 Novel Coronavirus (COVID-19) [Google Scholar]
- 22.Non-Emergent Elective Medical Services, and Treatment Recommendations. 2020. Available from: https://www.cms.gov/files/document/cms-non-emergent-elective-medical-recommendations.pdf [Last accessed on 2023 Jul 23]
- 23.Ostrom QT, Price M, Neff C, Cioffi G, Waite KA, Kruchko C, et al. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2015-2019. Neuro Oncol. 2022;24(Suppl 5):v1–95. doi: 10.1093/neuonc/noac202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price SJ, Joannides A, Plaha P, Afshari FT, Albanese E, Barua NU, et al. Impact of COVID-19 pandemic on surgical neuro-oncology multi-disciplinary team decision making: A national survey (COVID-CNSMDT Study) BMJ Open. 2020;10:e040898. doi: 10.1136/bmjopen-2020-040898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qi X, Keith KA, Huang JH. COVID-19 and stroke: A review. Brain Hemorrhages. 2021;2:76–83. doi: 10.1016/j.hest.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samer Zammar SS. COVID-19 and Neurosurgery. 2020. Available from: https://www.aans.org/en/patients/neurosurgical-conditions-and-treatments/covid-19-andNeurosurgery [Last accessed on 2023 Mar 07]
- 27.Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115:3–8. doi: 10.3171/2011.2.jns10998. [DOI] [PubMed] [Google Scholar]
- 28.Sarpong K, Dowlati E, Withington C, Chesney K, Mualem W, Hay K, et al. Perioperative coronavirus disease 2019 (COVID-19) incidence and outcomes in neurosurgical patients at two tertiary care centers in Washington, DC, during a Pandemic: A 6-month follow-up. World Neurosurg. 2021;146:e1191–201. doi: 10.1016/j.wneu.2020.11.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarwan G, Mubarak T, Puello P, Brisman M, Grewal J. Negative impact of COVID-19 upon primary brain tumor care. Cureus. 2021;13:e17800. doi: 10.7759/cureus.17800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.SEER Cancer Statistics Review 1975-2018 [database on the Internet] 2021. Available from: https://seer.cancer.gov/archive/csr/1975_2018 [Last accessed on 2023 Mar 06]
- 31.SEER Surveillance, Epidemiology, and End Results Program (SEER Database) Available from: https://seer.cancer.gov/statfacts/html/brain.html [Last accessed on 2023 Mar 05]
- 32.Sud A, Jones ME, Broggio J, Loveday C, Torr B, Garrett A, et al. Collateral damage: The impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann Oncol. 2020;31:1065–74. doi: 10.1016/j.annonc.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tai DB, Shah A, Doubeni CA, Sia IG, Wieland ML. The disproportionate impact of COVID-19 on racial and ethnic minorities in the United States. Clin Infect Dis. 2021;72:703–6. doi: 10.1093/cid/ciaa815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weller M, Preusser M. How we treat patients with brain tumour during the COVID-19 pandemic. ESMO Open. 2020;4(Suppl 2):e000789. doi: 10.1136/esmoopen-2020-000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wen PY, Weller M, Lee EQ, Alexander BM, Barnholtz-Sloan JS, Barthel FP, et al. Glioblastoma in adults: A Society for NeuroOncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020;22:1073–113. doi: 10.1093/neuonc/noaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19. 2020. Available from: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 [Last accessed on 2023 Mar 07]
- 37.Wiesner SM, Freese A, Ohlfest JR. Emerging concepts in glioma biology: Implications for clinical protocols and rational treatment strategies. Neurosurg Focus. 2005;19:E3. doi: 10.3171/foc.2005.19.4.4. [DOI] [PubMed] [Google Scholar]
- 38.Zarifkar P, Kamath A, Robinson C, Morgulchik N, Shah SF, Cheng TK, et al. Clinical characteristics and outcomes in patients with COVID-19 and cancer: A systematic review and meta-analysis. Clin Oncol (R Coll Radiol) 2021;33:e180–91. doi: 10.1016/j.clon.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


