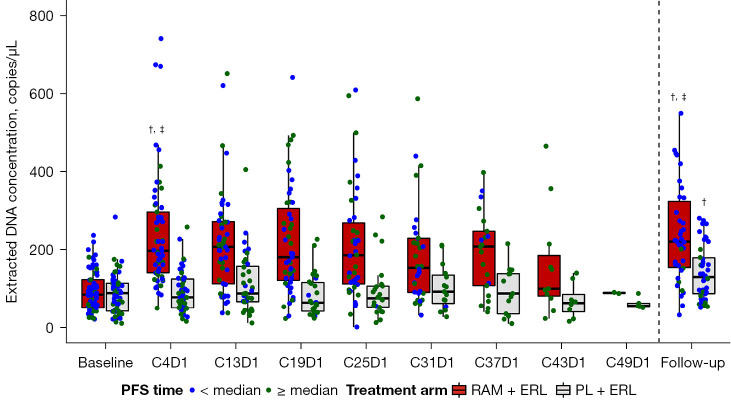
Figure 2.
Total cfDNA concentration by treatment arm (TR population, patients with a valid baseline sample). Population eligibility required the presence of a valid baseline sample only. Patients were dichotomized by median PFS time within treatment arm separately. Dots represent individual patient data. One patient had an extracted DNA concentration of 3,302.1 copies/µL at follow-up; this data point was removed from the plot. †, P for log-transformed data <0.0001 vs. baseline within treatment arm; ‡, P for log-transformed data <0.0001 RAM + ERL vs. PL + ERL at time point. C, Cycle; D, Day; PFS, progression-free survival; RAM, ramucirumab; ERL, erlotinib; PL, placebo; cfDNA, cell-free DNA; TR, translational research.