Fig. 1.
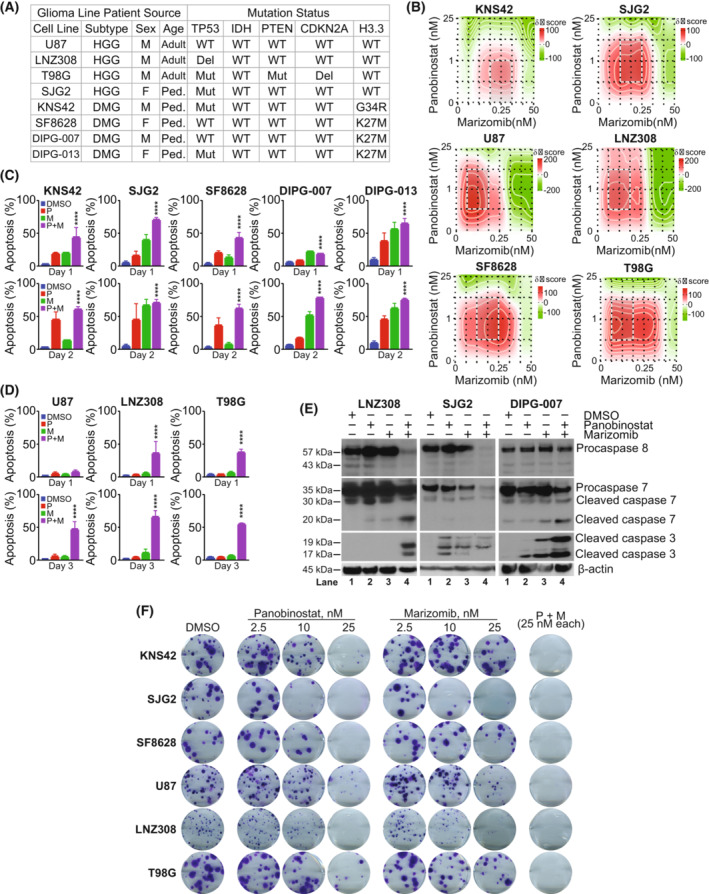
Cotreatment of panobinostat and marizomib promotes apoptosis in pediatric and adult glioma cell lines. (A) Genetic and biological characterization of the established glioma cell lines used in this study (HGG, high‐grade glioma; DMG, diffuse midline glioma; M, male; F, female; Ped, pediatric; WT, wild‐type; Del, deletion; Mut, mutation). The TP53 gene at chromosome 17p13.1, plays a critical role in the cell cycle, cellular responses to DNA damage, cell death, and differentiation. Isocitrate dehydrogenase 1 and 2 (IDH1 and IDH2) enzymes convert isocitrate to α‐ketoglutarate (α‐KG) through oxidative decarboxylation. IDH1/2 mutations introduce a gain‐of‐function activity to the enzyme that leads to accumulation of (+)‐2‐hydroxyglutarate (2‐HG). Phosphatase and TENsin homolog (PTEN), a gene located in the 10q23 region of chromosome 10 encoding for a 403‐aminoacid multifunctional protein, a crucial tumor suppressor, exhibits phosphatase‐dependent PI3K‐AKT–mTOR pathway activities to maintain cellular homeostasis. Cyclin Dependent Kinase Inhibitor 2A (CDKN2A), a tumor suppressor gene located at chromosome 9 blocks the progression of cell cycle from G1 to S phase. Recurrent mutations in H3 histone, family 3A (H3F3A), which encodes the replication‐independent histone 3 variant H3.3 led to amino acid substitutions at two critical positions within the histone tail (K27M, lysine at position 27‐to‐methionine; G34R, glycine at position 34‐to arginine; G34V, glycine at position 34‐to valine) involved in key regulatory post‐translational modifications. H3F3A mutations are highly prevalent in children and young adults. Histone 3 lysine 27‐to‐methionine (H3‐K27M) mutations most frequently occur in diffuse midline gliomas (DMGs). (B) Pediatric high‐grade glioma (KNS42, SJG2) or adult high‐grade glioma (U87, LNZ308, and T98G) and pediatric brain stem glioma (SF8628) were seeded in 96‐well plates in 75 μL of complete media. On the following day, cells were treated with an equal volume of panobinostat (0, 0.25, 1, 5, and 25 nm) or marizomib (0, 0.01, 0.05, 0.25, 1, 5, 25, and 50 nm) and their combination on cell proliferation in six glioma cell lines (KNS42, SJG2, U87, LNZ308, SF8628, and T98G). After 72 h of incubation at 37 °C, the number of viable cells was determined using a colorimetric cell proliferation assay kit as described in the Section 2. The degree of panobinostat and marizomib effect (synergistic or antagonistic or additive) was quantified using synergyfinder 2 (https://synergyfinder.fimm.fi), a stand‐alone web application for interactive analysis of drug combination. The data (n = 3) were analyzed using zero interaction potency (ZIP) model, which offers an increased power to differentiate between various classes of drug combinations for understanding their mechanisms of action toward clinical translation. The representative interaction landscape (heatmap) from three separate experiments for six different cell lines indicates that the combination of panobinostat and marizomib was able to achieve a higher effect (red‐shaded area) than the single agent. (C, D) KNS42, SJG2, SF8628, DIPG‐007, and DIPG‐013 (C); U87, LNZ308, and T98G cells (D) (1 × 105 per well) were plated in 6‐well microtiter plates. On the following day, cells were treated with panobinostat (25 nm, P), marizomib (25 nm, M), or both (P + M, 25 nm each) in combination. Control cells received an equivalent amount of DMSO (vehicle). After the indicated duration, the cells were stained with Annexin V and propidium iodide, and cell viability was assessed using flow cytometry as described in the Section 2. Values are represented as mean ± standard deviation of three separate experiments. Results were analyzed by Tukey's ANOVA (C, D, ****P < 0.0001, vehicle‐treated cells vs. combination of panobinostat and marizomib). (E) Logarithmically growing adult high‐grade glioma cells (LNZ308) were treated with panobinostat (25 nm) or marizomib (25 nm) or the combination of both (Pano + Mari, 25 nm each) for 72 h (left panel) and pediatric cells (SJG2 and DIPG‐007) for 48 h (middle and right panel). Twenty micrograms of protein were loaded on a sodium dodecyl sulfate‐polyacrylamide gel and probed with the indicated antibodies by western blotting. These experiments were performed at least three times, and a representative blot is presented. (F) Pediatric (KNS42, SJG2, and SF8628) and adult (U87, LNZ308, and T98G) cells were treated with indicated concentrations of panobinostat (2.5, 10, and 25 nm) or marizomib (2.5, 10 and 25 nm) or the combination of both (P + M, 25 nm each) for 24 h. Control cells received vehicle (DMSO). Clonogenic assay was performed as described in the Section 2. These experiments were performed at least three times, and a representative image is presented.
