Fig. 3.
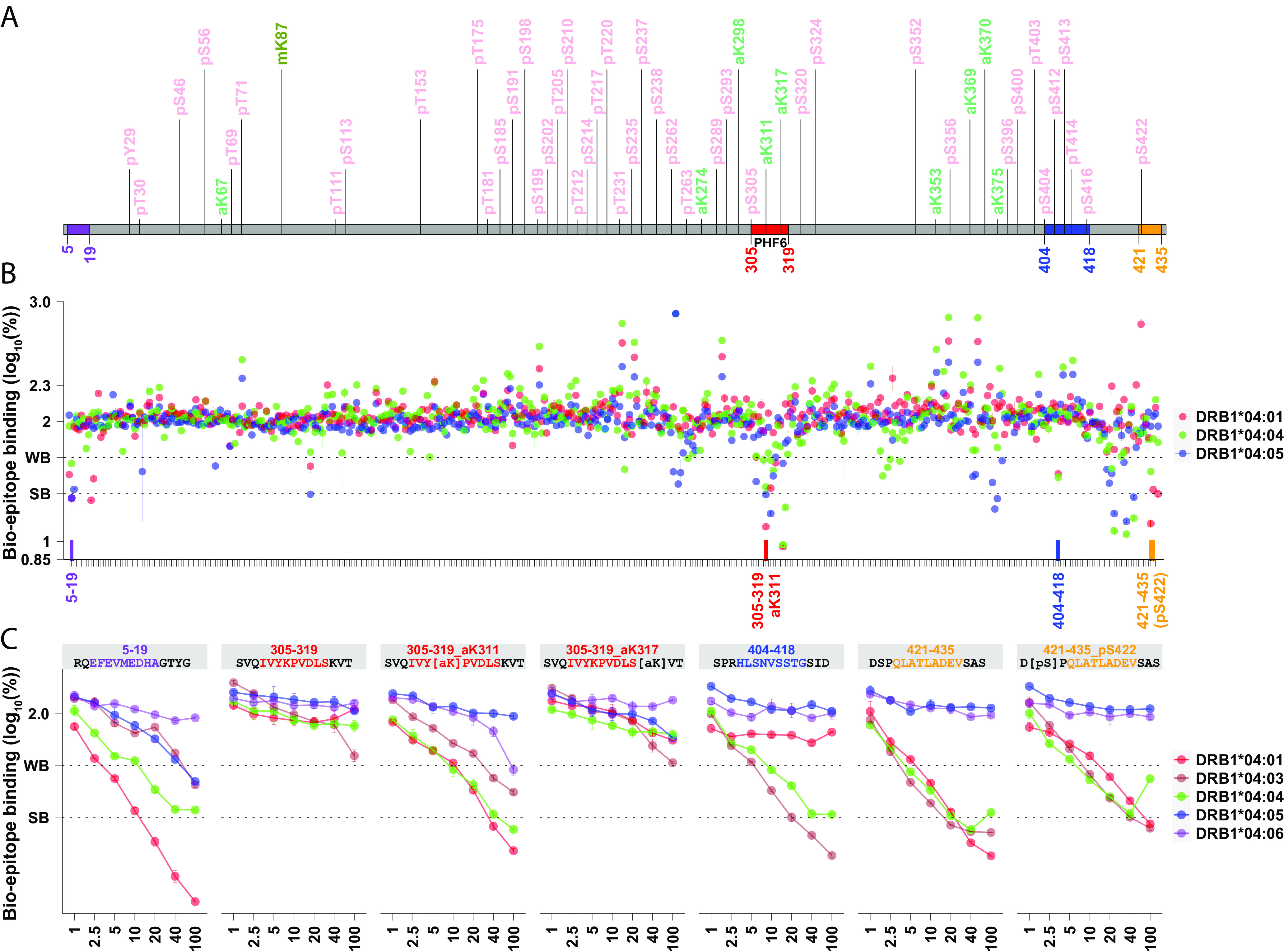
The pro-aggregation PHF6 region of tau binds HLA-DRB1*04 subtypes only when acetylated at K311. Fifteen-mer peptides (800 µM) encompassing the entirety of all tau isoforms (schematized in panel A), overlapping across 11 residues, were screened for HLA-DRB1*04:01, HLA-DRB1*04:04 and HLA-DRB1*04:05 binding (Methods), with and without common PTMs as reported by Wesseling et al. (32). Four regions (labeled in purple, red, blue, orange, panel B) containing strong HLA-DRB1*04 binders (log displacement <1 to 1.4, below 25% of baseline control) were further tested at various concentrations (panel C), showing three promising regions where binding was stronger with HLA-DRB1*04:04/ HLA-DRB1*04:01, intermediary with HLA-DRB1*04:03 and absent or weak with HLA-DRB1*04:05 and HLA-DRB1*04:06, a pattern similar to GWAS risk (Table 1). Among these candidate regions, PHF6 306VQIVY(acetylK)PVDLSK317 is the only one that strongly binds HLA-DRB1*04:01, HLA-DRB1*04:03, and HLA-DRB1*04:04 and only when posttranslationally modified at K311. This segment is well known to seed tau aggregation, and this process is increased in the presence of acetylK311. Outcompeting a biotinylated epitope (known binder) 75% and 50% is considered as strong binding (SB) and weak binding (WB), respectively. Predicted binding cores are highlighted correspondingly.
