Abstract
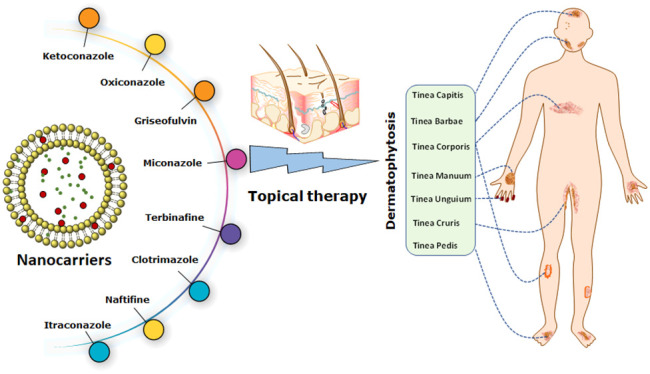
The most prevalent infection in the world is dermatophytosis, which is a major issue with high recurrence and can affect the entire body including the skin, hair, and nails. The major goal of this Review is to acquire knowledge about cutting-edge approaches for treating dermatophytosis efficiently by adding antifungals to formulations based on nanocarriers in order to overcome the shortcomings of standard treatment methods. Updates on nanosystems and research developments on animal and clinical investigations are also presented. Along with the currently licensed formulations, the investigation also emphasizes novel therapies and existing therapeutic alternatives that can be used to control dermatophytosis. The Review also summarizes recent developments on the prevalence, management approaches, and disadvantages of standard dosage types. There are a number of therapeutic strategies for the treatment of dermatophytosis that have good clinical cure rates but also drawbacks such as antifungal drug resistance and unfavorable side effects. To improve therapeutic activity and get around the drawbacks of the traditional therapy approaches for dermatophytosis, efforts have been described in recent years to combine several antifungal drugs into new carriers. These formulations have been successful in providing improved antifungal activity, longer drug retention, improved effectiveness, higher skin penetration, and sustained drug release.
1. Introduction
Dermatophytosis is a fungal illness caused by fungi (geophilic, zoophilic, or anthropophilic) that penetrate the skin’s outermost dead layer or keratinized tissues like hair and nails. The dermatophytes are classified into three distinct genera, specifically Trichophyton, Microsporum, and Epidermophyton.1 According to surveys conducted by the World Health Organisation, it has been found that approximately 25% of the global population is impacted by dermatophytes.2 Dermatophyte infections, often known as tinea infections, are the most prevalent type of infection seen all over the world.3
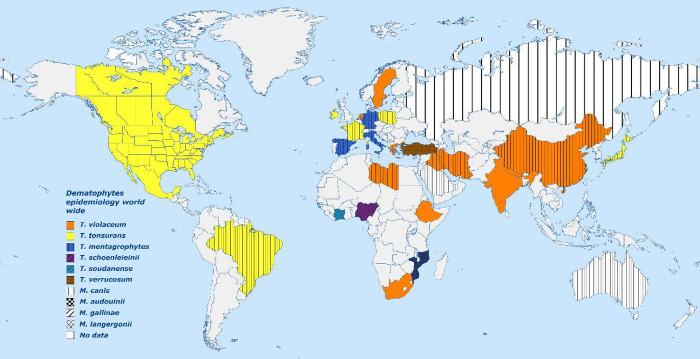
Approximately 40 species of dermatophytes have the potential to impact human health. T. rubrum, T. tonsurans, and M. canis are the most commonly occurring fungal species.4−6 The dermatophytes adapt their characteristics to new environments based on factors such as climate and socioeconomic standing.7 Prior to the middle of the 20th century, Epidermophyton floccosum, Microsporum audouinii, and Trichophyton schoenleinii were the predominant pathogens causing superficial fungal diseases; nowadays, they have been limited to a few underdeveloped countries. The prevalence of certain pathogens, such as T. rubrum, T. interdigitale, T. tonsurans, and M. canis, exhibited a consistent increase and emerged as the predominant species worldwide.8 Dermatophytes such as T. verrucosum, T. violaceum, and M. ferrugineum are prevalent in various regions of Europe, Asia, and Africa. Tinea rubrum is presently recognized as the primary causative agent of cutaneous and onychomycosis fungal infections on a worldwide basis. Tinea violaceum is predominantly prevalent in Eastern Europe, Africa, and Asia, whereas Microsporum canis is commonly found in numerous regions of Europe and Asia. Trichophyton tonsurans, on the other hand, is predominantly observed in the United Kingdom and North/South America, specifically in cases of tinea capitis.8−14 The illustration in Figure 1 displays various dermatophytosis pathogens and their corresponding geographical distribution worldwide.15
Figure 1.
Dermatophytes epidemiology on a worldwide basis. A solid color denotes that the identified Trichophyton is responsible for more than 85% of the cases in that country. Colorless hatching denotes that the identified Microsporum is responsible for more than 90% of cases in that nation. Both Trichophyton and Microsporum are significant causes of fungal infection in that nation, as evidenced by the combination of color and hatch patterns.
According to projections, this condition affects between 30 and 70% of persons asymptomatically, and its prevalence rises with age. Other factors that affect its epidemiology include climate factors, migration, and personal aspects including immunological status.2 These diseases are spread either directly by coming into contact with an infected person or animal, or indirectly by coming into contact with contaminated soil or termites.16 The primary mode of dermatophyte transmission is the perspiration of infected skin cells and hair. Transmission directly by contact is limited.1
The possible arthrospores or hyphae are deposited onto the surface of the susceptible host. Following the initial introduction into the host’s skin, the infection proceeds through a series of stages, including adherence, penetration, and retention, which are facilitated by specific favorable conditions. It is possible for certain fungi to adhere to specific hosts due to a wide variety of mechanisms and host factors, such as the ability to adapt to human biology, the number and activity of sweat glands in a specific part within the human body (as sweat exhibits an inhibiting effect on dermatophytes), ruptures in the skin barrier, mashed skin, and increased hydration.17 Limited information is currently available regarding the factors that promote the adherence of dermatophytes. The adherence of dermatophytes has been postulated to be mediated by proteases that are secreted by them. T. rubrum possesses the capability to attach to epithelial cells by virtue of carbohydrate-specific adhesins that are expressed on the surface of arthroconidia, which is the infectious agent.18 Following the adherence of arthroconidia to keratinized tissue, their growth and germination proceed in a radial manner, expanding in multiple directions.18,19 The proteases present in dermatophytes are responsible for the breakdown of the keratin network into oligopeptides or amino acids. Upon establishment, the spores undergo germination and subsequently penetrate the layer of the stratum corneum. This penetration is accompanied by the keratinases found in the dermatophytes. Fungal metabolic products diffuse through the malpighian layer, causing erythema, vesicle building, and pruritus.17 After the dermatophytes have invaded and contaminated the stratum corneum, the next phase is retention, during which they remain in the stratum corneum and rarely progress deeper into the epidermis than the surface and its extensions.20
Dermatophytosis is categorized based on the specific anatomical location of the infection. These classifications include tinea pedis for infections on the feet. Tinea cruris can be treated with infections in the groin area. Tinea corporis for infections on glabrous skin. Tinea barbae for ringworm of the beard and moustache. Tinea faciei for infections on the face. Tinea imbricata, which is a chronic superficial mycosis primarily caused by Trichophyton concentricum. Tinea capitis for infections on the scalp, eyebrows, and eyelashes. Tinea manuum for infections on the hands and Tinea unguium for infections on the nails. The site specific classification of fungal infection is illustrated in Figure 2.21 Similarly, Table 1 illustrates the examples of dermatophytosis as well as the location of the illness, the frequency of occurrence all over the world, and the clinical presentation.
Figure 2.
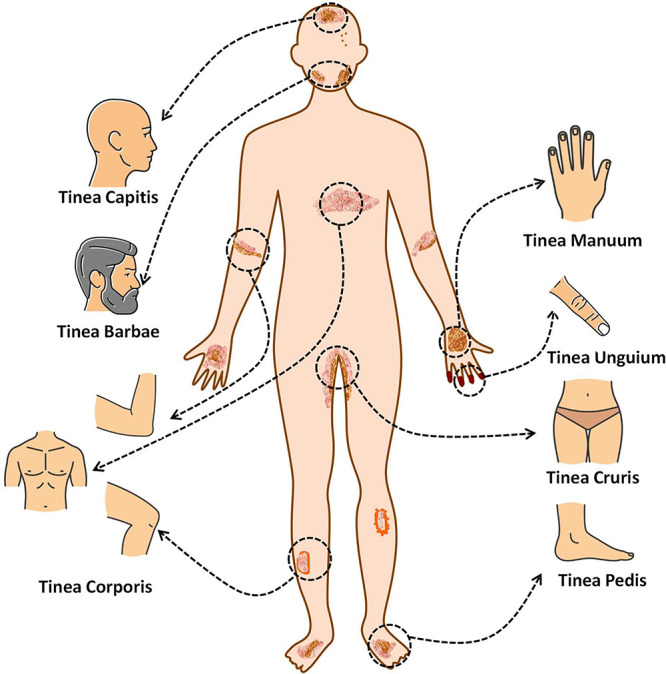
Specific form of dermatophytosis in humans along with the anatomic location of infection (the organs or tissues) that is targeted by the infection.
Table 1. Dermatophytosis Name along with Infection Site, Prevalence of the Disease Worldwide, and Clinical Manifestations of the Disease.
| S. No. | Dermatophytosis name | Infection site | Worldwide prevalence | Clinical manifestations | Ref |
|---|---|---|---|---|---|
| 1. | Tinea pedis | Feet | 4.2% for men and 1.7% for women | Long-standing, itchy, intertriginous dermatitis of the toes characterized by peeling, maceration, and fissuring | (22) |
| 2. | Tinea cruris | Groin | 20 to 25% of the world’s population | Red and itchy rash in warm and moist areas of the body | (23) |
| 3. | Tinea corporis | Glabrous skin | 0.6% of dermatophytosis | A well-demarcated, sharply circumscribed, erythematous, annular, scaly plaque with a raised leading edge, and scaling and central clearing on the body. | (24) |
| 4. | Tinea barbae | Ringworm of the beard and moustache | 5.8% of dermatophytosis | Swollen red patches, dry, scaly rashes, severe itchiness, patches of hair loss (alopecia). | (25) |
| 5. | Tinea faciei | Face | 0.5% of dermatophytosis | There are round or oval red scaly patches, often less red and scaly in the middle or healed in the middle. | (24) |
| 6. | Tinea capitis | Scalp, Eyebrows, and Eyelashes), | 8.7% of dermatophytosis | Swollen red patches. Dry, scaly rashes. Severe itchiness. Patches of hair loss (alopecia). | (26) |
| 7. | Tinea manuum | Hand | 0.1% of dermatophytosis | Itchy, round patches on the back of the hands | (24) |
| 8. | Tinea unguium | Nails | 0.8% of dermatophytosis | Long-standing, itchy, intertriginous dermatitis of the toes characterized by peeling, maceration, and fissuring | (24) |
The range of severity of these infections spans from cases that are mild or asymptomatic to those that have the potential to cause systemic infections that can be life-threatening. There exists a pressing necessity to improve the treatment of fungal infections. However, managing fungal infections poses a significant challenge. At present, topical formulations such as creams, gels, and lotions that incorporate antifungal agents are widely employed for the management of cutaneous fungal infections. Topical treatments exhibit localized action and entail less adverse effects compared to orally administered antifungal medications. Topical antifungal preparations have two main effects: either they kill the fungus (fungicidal) or they prevent them from growing (fungistatic).27 The likelihood of interactions with other drugs is minimal with topical formulations, unlike oral antifungal medications which are more prone to such interactions.28 Notwithstanding the efficacy of antifungal preparations, namely creams, gels, and lotions, there exists a possibility of encountering untoward outcomes, including cutaneous erythema, a vascular response characterized by skin reddening due to augmented blood flow, as well as stinging and a sensation of burning upon topical administration.29
As a result, researchers in the pharmaceutical domain have investigated diverse nanocarrier mechanisms to tackle these prerequisites and deliberations for administering antifungal medications via topical means.30 Nanocarriers have the capability to effectively target hair follicles and accumulate within the intercellular spaces of the stratum corneum, where they can integrate with the lipid matrix and interact with skin lipids. Nanocarriers present a multitude of benefits in comparison to traditional delivery systems for transdermal administration.31 Furthermore, nanocarriers have the capability to maintain drug release over an extended period, thereby mitigating adverse effects and minimizing the frequency of antifungal drug dispensation.32
The main objective of this Review is to examine the limitations associated with the transdermal delivery of antifungal medications. In order to enhance the therapeutic efficacy and circumvent the limitations associated with conventional therapeutic modalities for dermatophytosis, various endeavors have been documented to amalgamate multiple antifungal agents within novel carriers. The aforementioned formulations have demonstrated efficacy in enhancing antifungal activity through the reduction of drug resistance, mitigation of adverse effects, prolonged drug retention, increased effectiveness, enhanced skin permeation, and sustained drug release.
2. Epidemiology of Dermatophytosis Geographically
Dermatophytosis, a frequently occurring superficial infection, exhibits a worldwide distribution, with a higher incidence in tropical and subtropical areas, attributed to the elevated levels of temperature and humidity.33 Dermatophytosis is believed to impact around 20–25% of the global population.34 Changes in the epidemiological patterns of the pathogens may be associated with the emergence of persistent and treatment-resistant cases of dermatophytosis. The aforementioned phenomenon has resulted in the development of dermatophyte genotypes that exhibit heightened levels of virulence and pathogenicity. Furthermore, the emergence of drug-resistant species has been attributed to the inadequate administration of potent antifungal drugs.35,36 According to a study, the prevalence and variety of dermatophytosis infections have increased in recent years. This can be attributed to shifting migration and tourism patterns, socioeconomic situations, and increased contact with animals. As a result, endangered species have been identified in several nations.37
It is noteworthy that in developing nations, there exists a dearth of extensive epidemiological data due to the limited research that is dedicated to investigating the etiology of dermatophyte infections. Hence, it is plausible that the prevalence of dermatophytosis in a given country may not be accurately represented by the findings obtained from specific locations within that country.34,38
In various parts of the globe, people are infected with dermatophytes in a variety of different ways, which reflects the varying geographic distributions of dermatophytes. The examination of the presence of these fungi is highly significant in the process of diagnosing, treating, and differentiating the condition from other clinical skin illnesses. T. rubrum is the most common species of Trichophyton isolated from human skin, followed by T. mentagrophytes. This was proven abundantly evident in Europe, where a high rate of T. rubrum infection was documented, but in Asia, a greater incidence of T. mentagrophytes was seen.39 The conditions of dermatophytosis vary greatly from region to region and even within the same country.
2.1. America
Dermatophyte Survey Committee of the Medical Mycological Society of the Americas performed an epidemiological survey on dermatophytosis cases in the US from 1993 to 1995 and published it in 1998. The reports of the survey showed that T. tonsurans was the most prevalent causative agent (44.9%), followed by T. rubrum (41.3%).23
However, the result of another survey performed at the Centre for Medical Mycology in Cleveland, Ohio from 1995 to 2002 showed that dermatophytosis due to T. rubrum appreciably increased from 37% to 47% in between 1999 to 2002 unlike cases of T. tonsurans which decreased from 32% to 17.9%. This trend has indicated that T. tonsurans have expended during the 1950s from Central America and the Caribbean to the southwest part of the United States (US) from Central America. Moreover, mycotic infections are more frequently observed in the black African American population.40
2.2. Middle East
Geographical regions cause great variations in the prevalence of dermatophytosis in the Middle east. According to the publications, tinea corporis is the leading form of tinea infection in Iran.41 A study was done in Mashhad, northern Iran; as expected, the result showed that tinea corporis is approximately 33.1% of total tinea infection followed by tinea capitis (32.5%) and tinea pedis (3.4%). T. verrucosum was found to be the primary pathogen followed by T. violaceum and T. mentagrophytes, and a majority of tinea capitis infections were caused by T. violaceum (27%). After 10 years, in 2013, Tinea pedis (43.4%) and Tinea unguium (21.3%) were the most often seen infection in Tehran, and T. interdigitale became the leading pathogen.42 Around 2004, in Lebanon, tinea unguium was the chief form of tinea infection, with a prevalence of 44.2% of total dermatophytosis followed by tinea corporis (43.2%). Most active species was T. tonsurans (54.8%), followed by T. mentagrophytes, M. canis, T. rubrum, and T. verrucosum.43
In between 2003 and 2005, a survey was done in the Riyadh Military Hospital situated in Saudi Arabia, and the result revealed that 40.3% of the total dermatophytosis was onychomycosis, and tinea capitis was the second most prominent with 21.9% of the total cases. T. mentagrophytes and M. canis were acting as a principal causative agent.44
Sahin et al. published a survey report on a randomized study conducted in the remote area of Duzce, Turkey. The result showed that tinea pedis (49.1%) and tinea unguium (35.8%) were the major reason for dermatophytosis, and the principal causative agent was T. rubrum followed by T. mentagrophytes.33
Tinea pedis accounted for 45.1% of all dermatophytosis infections among 67 Iraqi patients, followed by tinea manuum (22.2%), tinea capitis (11.8%), tinea corporis (7.8%), tinea unguium (5.7%), and tinea faciei and tinea cruris (3.57%).45
2.3. Africa
It is difficult to estimate the real data of dermatophytosis in Africa because of the lack of published information regarding this topic. Dermatophytosis is not so uncommon in underdeveloped countries of Africa, but it generally remains undetected due to poor knowledge of it. It is difficult for the general population of Africa to bear treatment and medicine costs; thus, they commonly ignore this disease. Tinea capitis represents the most frequently encountered dermatophytosis in Africa, thus leading to a predominant focus on tinea capitis in the existing literature. Prevalence of fungal species responsible for the disease changes according to the geographical region.46 According to reports and publications, primary dermatophytosis in Nigeria is tinea capitis, and its major victims are children. T. soudanense and T. tonsurans are the pathogens in Abia state Nigeria, and in Anambra state, M. audouinii is the principal agent. Because of having a younger population (according to the report published in 2001, 44% of the total population is younger than 15 years) in Ethiopia, East Africa, a high incidence of tinea capitis caused by T. violaceum has been reported. The same trend has been observed in a survey conducted between 2009 and 2010 in Botswana.47 In the Egyptian governorate of Menoufia, tinea capitis was the most common clinical form of dermatophytes among students.48
2.4. Asia
In Asian nations, dermatophytes account for 40 to 48% of cases, with yeasts being responsible for 43–46% skin infections and nondermatophyte molds responsible for 8–11% of infections.49 A survey was conducted in 16 dermatological clinics of Japan involving 63,029 patients, it was found that tinea pedis was the most active type of dermatophytosis, tinea unguium was the second one.13 When a study was conducted in a rural area of South India, it revealed that tinea corporis and tinea capitis were the most active forms of tinea infection, followed by the cases of tinea cruris. As expected from the worldwide trend, T. rubrum (58.9%) was the principal causal agent, followed by T. mentagrophytes (24.6%). But these statistics are changing drastically; now the prevalence of T. mentagrophytes has increased from 20% to 90% in the past 15 years. It has been reported that 78% of the total patients reaching a dermatologist for skin lesions are suffering from dermatophytosis, this number is equal to the 20–25% of global prevalence.50 The Eastern Province of Saudi Arabia is particularly vulnerable to fungal diseases like tinea corporis and tinea cruris owing to its location near the Arab Gulf.51 Out of 115 individuals diagnosed with dermatophytosis in Baghdad, 26.7% had tinea corporis, whereas just 3% had Tinea manuum.34 Tinea corporis was likewise found to be the most common form of the infection in India, accounting for 35.4% of cases, followed by Tinea cruris and Tinea capitis each accounting for 16.8% of cases.52
2.5. Europe
Dermatophytes such as Microsporum canis and Trichophyton verrucosum are the most commonly isolated dermatophytes in regions such as Southern Europe and Arabic countries. This dermatophyte is the most prevalent agent responsible for tinea capitis in children at the present time. The rise in the frequency of M. canis infection in Europe, specifically in nations adjacent to the Mediterranean, has resulted in a significant surge in the incidence rate over the past few years.53 A total 350 samples from 322 individuals were analyzed. Out of 100 samples, 90 patients (28.6%) tested positive by direct microscopy and/or culture. Among 63 positive cultures (18%), 17 (3%) were yeasts, 2 (3%) were molds, and 44 (69.8%) were dermatophytes. Trichophyton rubrum (mainly from onychomycosis) and Microsporum canis (from tinea capitis and tinea corporis in youngsters) were the most common dermatophyte species found. Nail samples, particularly those of women, were shown to contain yeasts (Candida species).54 About 40% to 68% of cases in Europe may be attributed to dermatophytes, whereas yeasts account for 21% to 55%.55 A retrospective study was conducted between 1985 and 2008 in Austria, and the results showed that 76.3% of total dermatophytes were zoophilic with M. canis being responsible for 84.4% of the total cases of Tinea corporis. But in Italy re-emergence of anthropophilic dermatophytes like M. audouinii, T. violaceum, and T. tonsurans has been observed over the last 20 years.56 In Germany, E. loccusum and M. audouinis were the commonest causative agents of tinea during the 1920s. But this trend took a turn during the 1950s when T. rubrum became the most frequently observed dermatophyte in Europe chiefly responsible for tinea pedis and T. unguium.57 Mycology Reference Laboratory, Bristol, United Kingdom (UK), conducted a survey from 1980 to 2005 and found that T. rubrum was responsible for approximately 70% of total dermatophytosis in 2005, T. interdigitale was the second most prevalent dermatophyte accountable for causing approximately 20.8% of total cases of dermatophytosis.42
3. Factors Associated with Dermatophytes Infection
Apart from enzymes, several other factors have been associated with an increased incidence of dermatophytosis. These include elevated temperatures and humidity in tropical and subtropical areas as well as the geographical location, with a higher prevalence of the infection in rural regions compared to urban ones.58 Patients diagnosed with diabetes serve as a noteworthy illustration of how chronic ailments and disorders can potentially facilitate the transmission of infectious diseases. The incidence of tinea infections is significantly higher in developed countries due to the administration of immunosuppressive drugs and the higher prevalence of conditions such as Acquired Immune Deficiency Syndrome (AIDS), as opposed to infections in individuals residing in impoverished socioeconomic conditions.59 Additionally, the utilization of antibiotics and steroid medications, in conjunction with residing in a communal setting, may increase the likelihood of contracting an infection.45
In humans, the incubation period for dermatophytosis is normally between 1 and 2 weeks before symptoms of the disease become visible. The humid and warm conditions that are typical of tropical places are ideal for the propagation of the disease.48 Dermatophyte infections are linked to a number of risk factors, including a lack of cleanliness and perspiration that happens as a consequence of engaging in strenuous outdoor activities when temperatures are high. Dermatophytes are a type of fungus that can cause infections of the skin.51 The shifting epidemiology of dermatophytosis was influenced by a variety of factors, including travel, socioeconomic status, use of antifungal medication, and immunosuppressive status among others.34,60
4. Treatment Strategies for Superficial Fungal Infections
In the past few years, there has been a significant lack of attention paid to research pertaining to the treatment of dermatophytosis. This is concerning given the increasing prevalence of cutaneous dermatophytosis worldwide, particularly in tropical regions. As a direct consequence of this, the illness can still be found in a significant number of people all over the world and provides a complex therapeutic challenge to practitioners of medicine.23 There exists a variety of treatment modalities that can be utilized for the management of dermatophytosis. Although these medications demonstrate promising clinical cure rates, they are also linked to notable limitations, such as the emergence of antifungal drug resistance and adverse reactions. The azole-derived compound is a frequently employed antifungal approach owing to its extensive range of effectiveness, chemical stability, and superior oral bioavailability.61 Antifungal drugs, including polyenes, azoles, allylamines, echinocandins, and other classes of drugs, can be classified according to their respective mechanisms of action. The modes of action of these classes are as follows:
-
1)
Polyenes bind to ergosterol: Instead of stopping an enzyme from working, it binds to ergosterol, the main sterol in fungus membranes, which disrupts membrane function enough to let cellular contents leak out (amphotericin B).62
-
2)
Azole derivatives: An enzyme termed 14-lanosterol demethylase, which is essential for the biosynthesis of ergosterol, a vital component of fungal cell membranes, is inhibited by azole medications. Azole derivatives like ketoconazole, fluconazole, itraconazole, and voriconazole interfere with the synthesis of ergosterol by inhibiting this enzyme, compromising the viability and integrity of fungal cell membranes.62
-
3)
Inhibition of squalene epoxidase: The allylamine class, which includes terbinafine and naftifine, works by blocking the squalene epoxidase enzyme during the initial stages of fungal ergosterol production.62
-
4)
DNA and RNA synthesis inhibitors: Flucytosine is an antifungal medication that works by preventing fungal cells from synthesizing DNA and RNA. Inside the fungal cell, it is transformed into fluorouracil, a metabolite that interferes with the normal synthesis of nucleic acids and prevents the growth and replication of the fungus.62
-
5)
1,3-β-Glucan synthase inhibitors: Echinocandins belong to this class of antifungal drugs. They work by inhibiting the activity of the enzyme 1,3-β-glucan synthase, which is responsible for the synthesis of a specific component of the fungal cell wall called β-glucan. By blocking the synthesis of β-glucan, echinocandins weaken the fungal cell wall, causing cell lysis and death. These different classes of antifungal drugs target various aspects of fungal cell structure and function, providing a diverse range of treatment options for fungal infections.62
-
6)
Inhibition of C-14 sterol reductase and C-11 sterol Isomerase by morpholines class.62
-
7)
Inhibition of chitin synthase: The fungal cell wall, which is mostly made up of chitin, glucans, mannans, and glycoproteins, is important for adhesion and for fungi to cause disease. It also acts as a protective shield, preventing molecules from reaching the plasma membrane. The primary mechanisms that antifungals which target the cell wall function are by stopping the production of chitin and -glucan.63
-
8)
Inhibition of Heat Shock Protein 90 (Hsp90): The Hsp family has a molecular helper called Hsp90. Pathogenic microorganisms in the host are able to stay alive because they make these proteins in reaction to toxic conditions. Hsp90 could be a target for antifungal therapy because it is linked to fungal pathogenesis, phase change in dimorphic fungi, and resistance to antifungal drugs.64
-
9)
Inhibition of microtubules assembly: Griseofulvin inhibits the formation of microtubules. It is understood that griseofulvin disrupts the intercellular synthesis of microtubules and prevents fungi from going through mitosis.65
-
10)
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) leading to cell death: Amphotericin B, miconazole, and ciclopirox cause the production of ROS and RNS, resulting in cell death.65
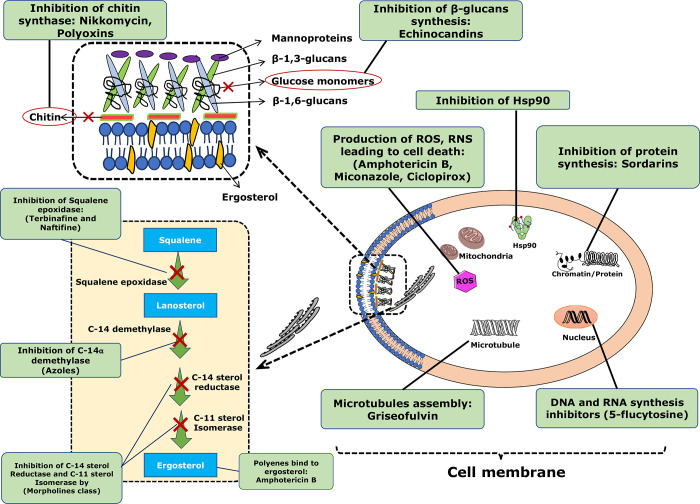
In addition to their ergosterol inhibitory properties, azoles have also been found to inhibit enoyl acyl carrier protein reductase, which has been associated with antibacterial activity. Triazole compounds exhibit greater efficacy against fungi, bacteria, and tumors in comparison to other azole derivatives.66 The mechanism of action of azoles as well as other fungal class drugs is illustrated in Figure 3.
Figure 3.
Mechanism of action of antifungal agents, including their respective target sites as follows: (1) Polyenes bind to ergosterol: Instead of stopping an enzyme from working, it binds to ergosterol, the main sterol in fungus membranes, which disrupts membrane function enough to let cellular contents leak out (amphotericin B); (2) azole derivatives that inhibit the 14a lanosterol demethylase (ketoconazole, fluconazole, itraconazole, and voriconazole); (3) Inhibition of Squalene epoxidase: Terbinfine and Naftifine; (4) DNA and RNA synthesis inhibitors (flucytosine); and (5) 1,3-β-glucan synthase inhibitors: Echinocandins inhibits the activity of the enzyme 1,3-β-glucan synthase; (6) Inhibition of chitin synthase by Nikkomycin, Polyoxins; (7) Inhibition of Heat Shock Protein 90 (Hsp90); (8) Inhibition of microtubules assembly (Griseofulvin); (9) Inhibition of C-14 sterol reductase and C-11 sterol Isomerase by morpholines class; (10) ROS, RNS leading to cell death: Amphotericin B, miconazole, and ciclopirox cause the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS), resulting in cell death.
4.1. Azoles
The largest group of antifungals is azoles. Currently, three generations of azoles are used clinically to treat dermatophytosis. The first-generation azoles have an imidazole in their ring structure and are mostly employed topically (with the exception of Ketoconazole; KTZ) due to their low oral absorption and severe systemic toxicity. Instead of an imidazole framework, the second and third generations of azoles have a triazole ring in their chemical structures.40 “Triazoles” have a more extensive range of action when compared to imidazoles. In addition, they are safer, have improved oral bioavailability, and have pharmacokinetic (PK)/pharmacodynamic (PD) properties.43 Examples of second-generation azoles include itraconazole (ITR) and fluconazole (FLU), whereas third-generation azoles include posaconazole, voriconazole, and isavuconazole.40 The C-14 demethylation phase of the ergosterol synthesis process is where the azoles are most effective. This is an oxidative process that takes place over the course of three stages and is mediated by the 14-lanosterol demethylase (P-450DM) enzyme.44 The interaction that results from the nitrogen atom of azoles binding to the iron heme of P-450DM is described here. This disruption of the pathway and accumulation of 14-methylated sterols disrupts the “bulk” function of ergosterol, which in turn increases the plasma membrane’s permeability to further damage and modifies the activity of membrane-bound enzymes, most notably those involved in nutrient transport and chitin synthesis.33Table 2 includes some examples of commonly used dosage regimens for the treatment of fungal infections.
Table 2. Standard and Modified Treatment Regimens for Fungal Infectiona.
| S. No. | Drugs | Target fungi | Mechanism of action | Ref |
|---|---|---|---|---|
| 1. | Fluconazole | Histoplasma, Blastomyces, and Coccidioides | Fluconazole interacts with 14-demethylase, a cytochrome P-450 enzyme responsible for catalyzing the conversion of lanosterol to ergosterol | (67) |
| 2. | Ketoconazole | blastomycosis, candidiasis, coccidioidomycosis, histoplasmosis, chromomycosis, and paracoccidioidomycosis | Ketoconazole works as an antifungal agent by inhibiting the cytochrome P-450 14α-demethylase enzyme. This enzyme is responsible for inhibiting the biosynthesis of triglycerides and phospholipids by fungi. | (68) |
| 3. | Itraconazole | Aspergillus species | Itraconazole acts by inhibiting the fungal cytochrome P-450 dependent enzyme lanosterol 14-α-demethylase. When this enzyme is inhibited, it blocks the conversion of lanosterol to ergosterol, which disrupts fungal cell membrane synthesis. | (69) |
| 4. | Terbinafine | Trichophyton species, Microsporum canis, Epidermophyton floccosum, and Tinea species | Its inhibition of fungal membrane production and ergosterol synthesis. | (70) |
| 6. | Clotrimazole | Candida albicans | Clotrimazole thereby inhibits the biosynthesis of ergosterol in a concentration-dependent manner by inhibiting the demethylation of 14 alpha lanosterol. | (71) |
| 8. | Miconazole | Candida spp., Trichophyton spp., Epidermophyton spp., Microsporum spp. | The primary mechanism of action is through inhibition of the CYP450 14α-lanosterol demethylase enzyme, which results in altered ergosterol production and impaired cell membrane composition and permeability, which in turn leads to cation, phosphate, and low molecular weight protein leakage. | (72) |
| Newer
Antifungal Therapy | ||||
|---|---|---|---|---|
| S. No. | Immune Therapy | Target fungi | Mechanism of action | Ref |
| 1. | T cell therapy | Aspergillus | Aspergillus-specific CD4+ T cells protect immunosuppressed host from aspergillosis infection | (73) |
| Invasive fungi | Developed CD4+ responses | (74) | ||
| Invasive fungi | Triggering C-type lectin dependent pathways | (75, 76) | ||
| Aspergillus and C. albicans | Developed Th1 memory response | (73) | ||
| 2. | Gene therapy | A. nidulans | Boosting NADPH function and the NET formation | (77, 78) |
| Aspergillosis | Boosting NK cell responses | (79) | ||
| Invasive fungi | Human recombinant IFN-γ fine-tune the immune responses | (80) | ||
| 3. | Cytokine therapy | Cryptococcus spp. | High levels of IFN-γ in cerebrospinal fluid reinforce the immune response to cryptoccal infections | (81, 82) |
| C. neoformans | 18B7 mAb attached to the polysaccharide capsule of C. neoformans and triggers the optimum immune responses | (83, 84) | ||
| 4. | Neutrophil and granulocyte therapy | Candida spp. | Granulocyte infusion | (85) |
| Invasive fungi | Phagocytic activity by different types of killer cells such as neutrophils | (86) | ||
| 5. | Antibody therapy | IFIs especially C. albicans | Efungumab attached to the HSP90 fungal shock protein and AmB and boosts immune responses to fungi | (83) |
| 7. | Monoclonal antibodies | Candida albicans, Candida, Cryptococcus, Histoplasma, Paracoccidioides, Sporothrix | Complement proteins trigger this portion of the innate immune response, enhancing C3 receptor-mediated phagocytosis. Antibodies also catalyze. | (87) |
| 8. | Rezafungin | Candida albicans, Candida glabrata, Candida parapsilosis | Cell morphological changes cause osmotic instability, which leads to cell death and/or inhibition of cell proliferation when 1,3-β-d-glucans are depleted from the cell wall. | (88) |
| 9. | Oteseconazole | Fungal CYP51 | Oteseconazole is a fungicidal CYP51 inhibitor that belongs to the azole metalloenzyme class. | (89) |
| 10. | Ibrexafungerp | Aspergillus spp. | Inhibits biosynthesis of β-(1,3)-d-glucan in the fungal cell wall | (90) |
| 11. | Laser treatment | Superficial fungal infection | Fungus killed by thermal process (1064 nm laser). | (91) |
| 12. | Olorofim | Invasive fungal infections | Olorofim acts by inhibiting the enzyme DHODH. | (92) |
NADPH: Nicotinamide adenine dinucleotide phosphate; NK: Natural killer; NET: Neutrophil extracellular traps; IFN-γ: Interferon-gamma; DHODH: dihydroorotate dehydrogenase.
4.1.1. Topical Treatment
Topical antifungal agents are considered the primary treatment option for superficial dermatophytosis due to their high efficacy and minimal systemic adverse effects. Pharmaceutical compounds are developed into a multitude of delivery systems, such as topical creams, sprays, lotions, and gels.93 The effectiveness and depth of penetration are dependent on the specific location of involvement. Upon topical application, these substances can readily penetrate the stratum corneum and exert antifungal effects by either inhibiting fungal growth or inducing fungal cell death.94 The three primary categories of antifungal agents utilized in the management of dermatophytosis are azoles, polyenes, and allylamine/benzylamines, which are commercially accessible in traditional dosage formulations. Imidazole’s exhibit broad-spectrum antifungal properties against dermatophytosis of glabrous skin when used as monotherapy.95
However, there are currently topical combination products on the market that contain both imidazole and corticosteroid, which are designed to treat patients with inflammatory dermatomycoses. These products have been found to offer more effective and frequent relief from inflammatory symptoms as well as improved rates of mycotic healing.96
Itraconazole (ITR) is often known to be utilized effectively in the treatment of dermatophytosis during the course of the past three decades. Although the medicine has a favorable pharmacokinetic profile when applied to the skin, its oral bioavailability is quite poor, and it has a high degree of interindividual variability.97 ITR moves extremely quickly to the subcutaneous tissue, most likely through the sebum, where it accumulates to levels that are far higher than those found in plasma.98 Because of the strong keratin adherence, the levels may be maintained for up to three to 4 weeks after therapy has been stopped; however, this can vary depending on the body area that is being treated.98 Even though there has not been a clinical occurrence of ITR resistance in dermatophytosis as of yet, there have been infrequent reports of higher MICs. These findings are almost always connected with T. interdigitale,99 the causative agent of dermatophytosis. In one study, authors aimed to assess the in vivo effectiveness of terbinafine in comparison to lanoconazole and luliconazole for the topical management of dermatophytosis caused by Trichophyton mentagrophytes, utilizing a guinea pig model.100 A clinical study was conducted to evaluate the efficacy of a 1% griseofulvin spray formulation and the vehicle alone in treating experimentally induced Trichophyton mentagrophytes lesions on the forearms of 16 healthy volunteers. The study was conducted in a double-blind manner. Furthermore, the investigation also assessed the effectiveness of the identical composition in managing a group of 100 patients with tinea pedis instigated by various dermatophytes.101
4.1.2. Oral Treatment
The oral route for antifungal administration is mainly used in the treatment of widespread skin lesions, systemic fungal infection, and in a condition where topical antifungals become unresponsive for example topical formulations can be used for low-grade tinea capitis and onychomycosis, but in severe conditions, oral antifungals are primarily used.102 Five chief systemic antifungals present on the market are terbinafine, ketoconazole, itraconazole, griseofulvin, and fluconazole. Terbinafine is orally administered in a dosage of 250 mg/day for the treatment of dermatophytosis. It produces fast and enduring remissions in dry type tinea pedis and tinea cruris, as well as tinea corporis when taken for 2 weeks.103 Ketoconazole (KTZ), Itraconazole (ITR), and Fluconazole (FLU) are three systemic azoles that are commonly employed in the treatment of dermatophytosis. The clinical efficacy of KTZ was found to be superior to that of griseofulvin (GRI), which was the sole systemic antifungal agent used for treating dermatophytosis at the time. KTZ was able to address various challenges associated with GRI, such as extended treatment durations, frequent treatment failures, an unfavorable skin pharmacokinetic (PK) profile, and limited oral bioavailability.104,105 It gave the benefits of high keratin adherence as well as prolonged therapeutic levels in the systemic circulation (SC) for up to 10 days after therapy had been discontinued.106,107 Although there have been sporadic reports of high in vitro MICs, to the best of our knowledge, no clinical instance of resistance to KTZ has been recorded up to this point.99,108 On the other hand, due to the hepatotoxic nature of the medicine’s side effects, it has been banned in certain nations, and strict limitations and extreme care have been recommended in others, despite the fact that there are some people who say that this should not be the case. In addition, the drug has been linked to a number of deaths.109 It is still used as an effective topical therapy for superficial mycoses,109 and some physicians may occasionally use it as a reserve medicine for resistant dermatophytosis at a dosage ranging between 200 and 400 mg/day.105
Fluconazole (FLU) has a high bioavailability when taken orally, it quickly accumulates in the SC, and it reaches very high levels.110 However, after treatment is discontinued, there is a chance that the substance will rediffuse back into the circulation. This suggests that the avidity with which it was bound was not particularly strong. The elimination from the SC happens with a half-life that can range anywhere from 60 to 90 h. This is a slower process than the elimination from the plasma.110 Despite the fact that FLU is not specifically prescribed for dermatophyte infection, it has been shown to be beneficial in treating dermatophytosis, particularly tinea capitis. This is the case even though it is not the intended use of the medication.111 FLU was initially given at a dosage of 50 mg/day, but later on, in light of the skin pharmacokinetic features, a weekly dose of 150 mg was tested, and it was proved to be effective in studies.112−114
Itraconazole works effectively against tinea cruris and corporis, and in dry type tinea pedis.115 Fluconazole is also used for the treatment of dermatophytosis of skin and shows the great result when given in a dose of 50 mg/day for 2–4 weeks.116 Griseofulvin is active against Trichophyton, Epidermophyton, and Microsporum species and acts as a first-line drug in the treatment of tinea capitis. Ketoconazole works actively against yeasts, some systemic fungal infections, and dermatophytes, such as tinea cruris, tinea capitis, and tinea pedis. Besides, it eradicates tinea versicolor when given orally for 1 week.117
Various oral antifungals are available in suspension form to allow for easier dosing for children. However, the oral suspension may exhibit a different pharmacological profile from tablets/capsules.
4.1.3. Intravenous Treatment
Amphotericin B is the traditional treatment of choice for a majority of systemic mycosis. But in the case of superficial fungal infections, it is preferred only for the management of chronic mucocutaneous candidiasis and candida granuloma.118 Miconazole is a broad-spectrum antifungal agent and shows the satisfactory result in the treatment of chronic mucocutaneous candidiasis.119 Furthermore, Caspofungin is an antifungal agent that has received exclusive approval for intravenous administration. This medication is specifically indicated for the treatment of invasive aspergillosis in patients who have demonstrated resistance to amphotericin B and itraconazole. Furthermore, the drug caspofungin has been granted approval for the management of infections caused by Candida spp. as reported by Hashemian et al.120
5. Drawbacks of Conventional Dosage Forms
5.1. Drawbacks of Conventional Oral Formulations
Oral antifungals exhibit more serious adverse events as compared with topical formulations. Along with being costly, some of them can produce organ toxicity and show frequent drug–drug interactions.121 Griseofulvin, a generally used oral antimycotic agent, shows adverse effects like hepatotoxicity, photosensitivity, headache, nausea, and vomiting. Ketoconazole, along with hepatotoxicity, shows other side effects like impotence, hemolytic anemia, and abdominal pain.122
Most of the antifungals have limited water solubility; as a result, this leads to poor oral bioavailability and restricted formulation approaches which adds further complications in antifungal formulation development.123
5.2. Drawbacks of Conventional Topical Formulations
Despite being cheaper and safer than oral antifungals, topical preparation may show local irritation, redness, erythema, stinging, and burning sensation at the site of application. It becomes insufficient to use topical antifungals in the treatment of severe and extensive superficial skin infections. In onychomycosis, topical antifungals show inferior results than oral preparations due to their inability to cross the nail bed.124
It may show a poor response when not applied in an adequate amount. Conventional topical agents generally show poor bioavailability due to difficulty in penetration through the Stratum corneum which acts as a protective multicellular barrier.125
6. Advantages of Nanocarriers over Conventional Treatment
6.1. Antifungal Drug Resistance and Underlying Mechanism
The global prevalence of drug resistance to antifungal medications is a significant epidemic, with severe implications for patient care. This includes adverse effects on both physical and mental health as well as a decrease in overall quality of life.126 Antifungal resistance became prevalent only in the late 1990s. In recent times, however, its incidence has increased. The azole antifungal agents have been observed to be less effective against dermatophytes due to the development of resistance.127 In a similar way, the discovery of T. rubrum that is resistant to terbinafine has been reported.128 Additionally, indications of resistance to griseofulvin and other antifungal medications were observed.127
In addition, causative agents of dermatophytosis may acquire resistance to all kinds of antifungals. A few of the most important include (a) lowering the drug’s accumulation inside the fungal cell, (b) diminishing the drug’s target affinity, and (c) adjusting metabolism to nullify the drug’s antifungal impact. The molecular mechanisms underlying azole action can be broken down into four distinct types: (i) a decrease in azole affinity for its target; (ii) an increase in the number of copies of the azole target; (iii) a change in the ergosterol biosynthesis pathway as a result of azole action; and (iv) a decrease in azole accumulation within the cell. It was shown that several mechanisms of resistance are often coupled when extremely resistant tissue isolates were collected from patients receiving long-term medication.129
Nanocarriers are currently seeing widespread application as a potential solution to the problems outlined above. The incorporation of antifungals into nanoformulations results in enhanced therapeutic action and, in many instances, a sustained effect due to the stimuli-responsive release characteristics. It is feasible to achieve the target-specific delivery of antifungals by appropriately ligand-tagging the formulation. This results in a lower required dosage, which in turn results in fewer adverse effects.130 According to existing literature, nanoformulations have the potential to exhibit a wide range of antifungal activity, facilitate sustained drug release, minimize the need for frequent dosing, and offer a novel mechanism of action that may help to surmount antibiotic resistance.131 El Rabey et al. conducted a study wherein they observed that chitosan nanoparticles loaded with fluconazole exhibited inhibitory effects against C. albicans, C. parapsilosis, and C. glabrata, including drug-resistant strains.132 Kelidari et al. conducted a study wherein they formulated solid lipid nanoparticles loaded with voriconazole. The results indicate a decrease in the minimum inhibition concentration (MIC) for both the resistant and susceptible strains.133 In another study, Salehi et al. prepared caspofungin loaded gold nanoparticles. According to the author’s report, the nanoparticles that were prepared have demonstrated efficacy against resistant strains.134 Noorbakhsh et al. conducted a study to investigate the effects of silver nanoparticles (Ag NPs) both alone and in combination with antifungal drugs, specifically fluconazole and griseofulvin, on T. rubrum. The findings indicated that the activity of T. rubrum was inhibited by Ag NPs alone when administered at a concentration of 10 μg/mL. Nevertheless, the inhibitory effect exhibited by them was comparatively lower in contrast to griseofulvin (0.8 μg/mL) and higher than fluconazole (40 μg/mL). It is noteworthy that the antifungal efficacy of these drugs was augmented upon amalgamation with Ag NPs.135
6.2. Side Effects Associated with Antifungal Treatment
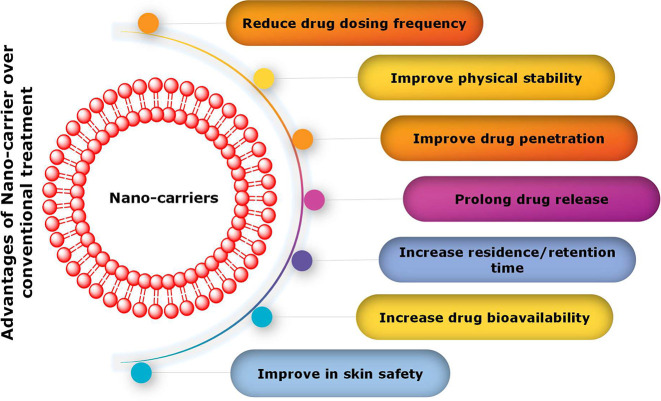
The adverse effects associated with antifungal therapy pose an additional challenge in the management of dermatophytosis. Certain individuals have reported instances of pruritus, erythema, or discomfort subsequent to the application of topical antifungal agents that comprise azoles, such as miconazole. The administration of econazole has been associated with pruritus, erythema, and a burning sensation, while the use of ketoconazole has been correlated with xerosis, irritation, seborrheic dermatitis, and a stinging sensation.136 Following the administration of Nystatin, some patients had uncomfortable side effects such as burning, rashes, itching, redness, and pustular eruption.137 It was stated that using tolnaftate could cause irritation to the user’s skin in some cases.57 The topical application of terconazole cream may result in cutaneous irritation or a sensation of burning.138 The use of systemic antifungal medications is also associated with serious adverse effects. The administration of triazoles such as fluconazole and itraconazole has been associated with a range of adverse effects, including but not limited to headache, dizziness, heartburn, alterations in taste perception, as well as more severe manifestations such as fatigue, anorexia, vomiting, paresthesia, urticaria, angioedema, dysphagia, pyrexia, and chills.139 Therefore, it is necessary to develop a system that has less adverse effects compared to the formulation that is currently in practice. In a study Hussain et al. prepared amphotericin B loaded nanoemulsion-gel for better antifungal activity. Based on the findings, it can be inferred that utilizing nano emulsion-gel as a delivery method is a cost-effective approach for safe and efficient localized administration of amphotericin B to treat fungal infections.140 The authors, Kassem et al., formulated a niosomal gel containing griseofulvin with the aim of treating tinea corporis. They conducted a comparative analysis of the efficacy of this formulation against liposomal gel and standard griseofulvin gel. A clinical trial was conducted involving 16 patients diagnosed with tinea circinata, wherein the efficacy of the niosomal gel was evaluated. The results indicated that the aforementioned gel demonstrated the most favorable outcomes in terms of both clinical and mycological cure over a treatment duration of 2.5 weeks. The investigators additionally noted that the niosomal gel comprising 1% griseofulvin exhibited efficacious therapeutic outcomes with negligible adverse reactions.141 The authors, Chen et al., presented an optimized formulation of solid lipid nanoparticles (SLNs) that may serve as a promising vehicle for the delivery of terbinafine. The aforementioned formulation exhibited enhanced permeability, thereby enabling a decrease in the frequency of dosage and mitigation of adverse effects. Consequently, the utilization of this particular mode of drug administration amplifies the safety, cost-efficiency, and tolerability of antifungal treatment.142Figure 4 illustrates several other advantages of drug delivery systems utilizing nanocarriers in comparison to conventional treatments.
Figure 4.
Potential advantages of utilizing nanocarriers in drug delivery systems when compared to conventional treatments.
7. Novel Drug Delivery System for the Treatment of Superficial Fungal Infections
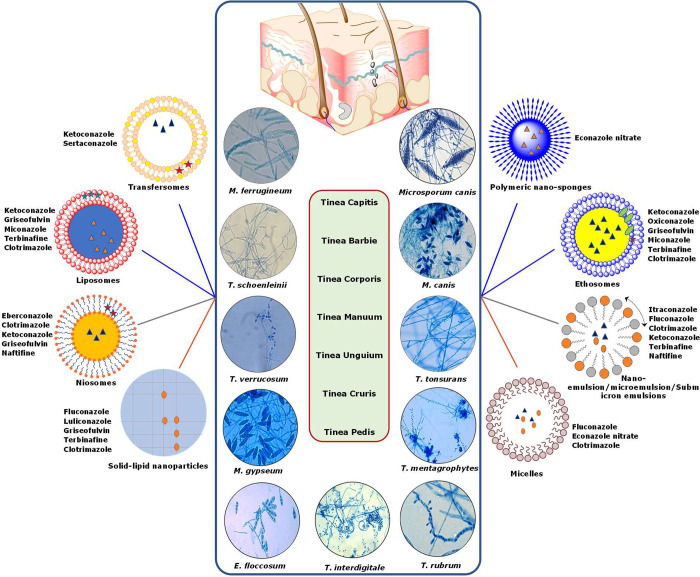
In the last ten years, the future of the pharmaceutical and biotechnology industries has been greatly improved by the use of nanotechnology to medicine. The use of nanomedicines in the treatment of superficial fungal infections has shown promising results. For the treatment of fungal infections, topical formulations based on conventional techniques, such as creams, lotions, sprays, and ointments, have not been able to accomplish active skin targeting and controlled release. By creating and manufacturing nanocarriers, novel drug delivery systems solve the problem with conventional drug delivery systems.143 Antifungal agents have been tested in a variety of nanoparticulate systems, including microemulsions, micelles, nanoemulsion or submicrometer emulsions, liposomes, niosomes, ethosomes, transfersomes, nanoparticulate carriers, and gelling systems, as shown in Figure 5. Antifungal nanotechnology has a number of benefits, including the capacity to deliver medication to a specific location, solve drug solubility or stability problems, and reduce adverse drug reactions.144 Additionally, nanodrug delivery systems are equipped to circumvent drug resistance pathways that are already in place. The numerous antifungals developed as nanomedicines for the treatment of superficial fungal infections are listed in Table 3 together with information about their preparation process, size, and intended use.145
Figure 5.
A wide range of nanoformulations incorporating azole antifungal medicines are being explored as potential treatments for dermatophytosis.
Table 3. Delivery of Antifungal Drugs Using Carriers for the Treatment of Superficial Fungal Infections, along with Details on Their Preparation, Size, and Intended Usage.
| S. No. | Active drug | Vesicular system | Particle size | Method of preparation | Comments | Ref |
|---|---|---|---|---|---|---|
| 1. | Eberconazole | Niosomes | 550–620 nm | Thin-film hydration method | The prepared niosomes showed high entrapment efficiency and release the drug in a controlled manner. | (174) |
| 2. | Ethosomes | 250–300 nm | Thin-film hydration method | The prepared EBZ loaded ethosomes showed excellent antifungal activity against C. albicans. | (145) | |
| 3. | Microsponge | 24.5–39.4 μm | Quasiemulsion solvent diffusion method | The prepared microsponge released the EBZ in a controlled manner and the skin irritation study on rats showed that the microsponges were nonirritant. | (175) | |
| 4. | Ethosomes | 200–300 nm | Thin-film hydration method | The prepared ethosomes have better penetrability and bioavailability. | (176) | |
| 5. | Terbinafine | Liposomes | 207–345 nm | Ethanol injection method | The liposome dispersion prolonged the release of drug and the findings revealed that the terbinafine-loaded liposomes could effectively treat fungal infection. | (177) |
| 6. | Ethosomes | 100–120 nm | Cold method | The prepared ethosomes penetrate across subcutaneous into dermis and epidermis. The ethosomes are biocompatible in nature confirmed by allergy and irritation test. | (178) | |
| 7. | Ufasomes | 376.5 nm | Thin-film hydration | The researchers formulated ufasomes containing terbinafine using glyceryl oleate as a base. These ufasomes demonstrated potent antifungal properties following a 5-day period. Consequently, it was demonstrated that ufasomes exhibited greater efficacy in comparison to the commercially available formulation. | (179) | |
| 8. | Solid lipid nanoparticles (SLNs) | 80–200 nm | Microemulsion method | The prepared SLNs improve the cost, safety, efficacy and tolerance of antifungal activity. | (142) | |
| 9. | Nanoemulsion | 20–200 nm | High-pressure homogenization | The prepared nanoemulsion when tested on wistar rat model, showed negligible skin irritation. The nanoemulsion gel showed good adhesion property on the skin. The prepared nanoemulsion is better treatment option for the treatment of fungal infection because of its good penetration ability. | (180) | |
| 10. | Clotrimazole | Ethosomes and Ultra deformable liposomes | 132–121 nm | Mechanical-dispersion | The most effective method for delivering clotrimazole dermally and transdermally is through ethosomes. | (161) |
| 11. | Surfactant-based nanovesicles | 479.60 nm | Ethanol injection | The increased antifungal impact of the optimized CLT formulation created in this study may represent a breakthrough in the effective ocular administration of CLT for the treatment of fungal keratitis. | (181) | |
| 12. | Liposomal/Niosomal | 4.111 mm | Thin lipid evaporation technique | The morphology of vaginal tissues at 24 h post dose was unaffected by liposomal or niosomal gels; this supports the safety of vesicle gels for vaginal administration. | (182) | |
| 13. | Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) | <1 μm | High-pressure homogenization | The SLN and NLC that were loaded with clotrimazole. Using these lipid nanoparticles as modified release formulations for 10 h for lipophilic medications. | (183) | |
| 14. | Ufosomes | <300 nm | Thin film hydration technique | Through the use of sodium oleate and cholesterol, clotrimazole-loaded ufosomes were created. The prepared ufosomes increase the skin bioavailability of clotrimazole. | (184) | |
| 15. | Griseofulvin | Ethosomes | 130 nm | Cold method | When employed in a 0.1% griseofulvin ethosomal formulation, GRF ethosomes were noncytotoxic, and a skin-adapted agar diffusion test verified GRF antifungal efficacy. | (185) |
| 16. | Deformable Membrane Vesicles (DMVs) | 284.6 nm | Thin-film hydration | An approach to lessen the burden of traditional oral formulations is the topical formulation of DMVs of griseofulvin. | (186) | |
| 17. | Liposomes | 16.53–28.63 nm | Thin film hydration | These topical liposomes included films may address the liposomal formulations’ instability issue and provide topical formulations that are simple to apply, particularly for long-term therapy. These formulations may be used to administer griseofulvin to superficial fungal infections, and in vivo tests are being done to determine how well they work against such infections. | (187) | |
| 18. | Ethosomes | 148.5 nm | The cold method | A praiseworthy option to lessen the bioburden associated with typical oral formulations might be dermal administration of griseofulvin using ethosomes. Because the proposed method delivers drugs site-specifically and with little adverse effects, it may result in a better disease remission. | (188) | |
| 19. | Niosomes | 3–5 μm | The thin film approach and the Ether injection method | The prepared niosomes enhanced oral bioavailability and longer drug release characteristics, the niosomal formulation may be a potential method of delivering griseofulvin. Niosomes may be effectively employed to increase the bioavailability and provide prolonged distribution of griseofulvin orally. | (189) | |
| 20. | Solid Lipid Nanoparticles (SLNs) | 117 nm | Hot microemulsion method | SLNs have an excellent skin penetration effect and might be a useful delivery method for griseofulvin. | (190) | |
| 21. | Naftifine (Naf) | Niosome Gel | 0.09–0.15 μm | Film hydration | A controlled delivery antifungal gel without alcohol that contains 1% (w/w) Naf.HCl was created. Over a 12-h trial period, the gel-maintained drug delivery and had acceptable stability. This study’s development of a Naf.HCl niosome gel may hold considerable promise for topical antifungal treatment. | (191) |
| 22. | Transethosomes | 50.20 nm | Thin-film hydration | To get over the stratum corneum barrier and improve naf skin penetration and deposition, a topical transethosomal system may be an option. | (192) | |
| 23. | Microemulsions | 7.34–11.17 nm | Water titration method | The created nanosized colloidal carriers might be used to administer naftifine topically in an efficient and secure manner. For cutaneous distribution, nanosized colloidal carriers of the microemulsion type are loaded with naftifine. | (193) | |
| 24. | Miconazole | Ethosomes | - | Cold method | Compared to liposomes and plain ointment, the Ethosomal reservoir system supplied the medicine (miconazole nitrate) at a regulated pace over 12 h, minimizing the harmful effects brought on by the drug. Thus, it might be concluded that the ethosomal system has superior sustained activity and skin penetration compared to liposomes. As a result, the ethosomes system is appropriate for the long-term topical distribution of miconazole nitrate. | (194) |
| 25. | Liposomes | 161–182 nm | The traditional rotational evaporation sonication process | The development of ultra flexible vesicles for topical and transdermal distribution opens up new possibilities for the current, well-regulated topical treatment of fungal infection. | (195) | |
| 26. | Bilosomes | 182–295 nm | Thin-film hydration | The prepared nanocarrier showed better therapeutic efficacy against C. albicans and A. niger as compared to pure miconazole. | (196) | |
| 27. | Nanoemulgel | 165 nm | The self-nanoemulsifying technique | The prepared nanoemulgel showed greater antifungal activity as compared to commercial product. | (197) | |
| 28. | Transfersomal gel | 368 and 931 nm | Thin-film hydration | Transfersomes are used for target delivery and to keep the drug’s release from the dose form consistent. In the form of transfersomes, miconazole nitrate may pass through the stratum corneum barrier and infiltrate the skin. | (166) | |
| 29. | Nano-Vesicle | <400 nm | Thin-film hydration | The ability to cure fungal infections by extending their duration in the epidermis, boosting their method of administration, and improving their effectiveness by using nanovesicles. | (168) | |
| 30. | Liposome | 110.8 and 539.5 nm | Thin-film hydration | Delivering miconazole, maintaining a consistent percentage of PG absorption in the vesicles (45.5%) in the PG concentration range of 2.5 to 10%, improving vesicle stability, and enhancing miconazole skin deposition with the least amount of skin permeability are all possible. | (198) | |
| 31. | Ketoconazole | Ethosomes | 169 nm | Thin-film hydration | The research indicated that the ethosomal formulation of ketoconazole had a greater entrapment efficiency and superior stability profile. The improved targeting of this medicine to the epidermal and dermal locations may be aided by the increased accumulation of ketoconazole through the ethosomal carrier inside the skin. | (199) |
| 32. | Ethosomes | 78.99 nm | Thin-film hydration | The prepared ethosomes showed 1.8-fold higher skin permeation and 5.6-fold high skin deposition as compared to drug suspension. | (200) | |
| 33. | Liposome | 141.6 nm | Thin-film hydration | Ketoconazole liposomal gel combined with neem extract has shown synergistic effects in the treatment of seborrheic dermatitis. | (201) | |
| 34. | Niosomal | 4.86–7.38 μm | Thin film hydration | The gel formulations with ketoconazole-loaded niosomes exhibited extended action compared to formulations with ketoconazole in non-niosomal form, which may have improved the antifungal effectiveness. | (159) | |
| 35. | Transfersomal | 126 nm | Homogenization hot method | The proposed formulation offers greater therapeutic effectiveness and gains from its nano size. The potential utility of an ultradeformable transfersomal system in the treatment of vaginal candidiasis is therefore indicated by the planned investigation. | (165) | |
| 36. | Liposomal | 70–150 nm | The traditional thin-film evaporation and hydration process | The innovative nanocarrier, which has the ability to improve skin target effect and create a micro drug-depot, may be used as an efficient skin targeting delivery system for KTZ, an antifungal agent used in local treatment. To target and deposit the lipophilic medication KTZ at the appropriate places, the lipid-based nanocarrier DEL offered great potential. | (156) | |
| 37. | Fluconazole | Nanoliposomal fluconazole | 88.9 nm | Thin film hydration | The main takeaway from this research is that nanoliposomal fluconazole, as opposed to more traditional versions of these medications, may accelerate the healing process of dermatophytosis. This research showed that nanodrugs are more effective in treating dermatophytosis and may be used as a future option to treat dermatophyte infections. | (202) |
| 38 | PLGA-nanoparticles | 110 nm | Coacervation method | The prepared nanoparticles showed a significant 16- and 64-fold enhanced antifungal activity (MIC 5 and 2.5 μg/mL) against C. albicans and C. auris, respectively, as compared to FLZ. | (203) | |
| 39. | Solid lipid nanoparticles | 292 and 500 nm | Modified high shearhomogenization and ultrasonication method | The high particle surface area and film formation in the produced FLZ-SLNs gel resulted in better interaction between FLZ and skin, which facilitated skin penetration. | (204) | |
| 40. | Itraconazole | Transethosomes | 207.4 nm | After further clinical research, these findings showed that the ICZ-loaded TEs gel would serve as a more effective alternative to commercially available formulations for the treatment of Trichophyton skin infections. | (205) | |
| 41. | Spanlastics (surfactant-based elastic nanovesicles) | 287 nm | Ethanol injection method | When the chosen spanlastic formulation was applied to the rabbit eye in an in vivo Draize test, there were no indications of acute ocular toxicity. Itraconazole-loaded spanlastics might be a promising nanosystems for ocular medication delivery. | (206) | |
| 42. | Oxiconazole nitrate | Solid lipid nanoparticles | 101.4 nm | Modified high shear homogenization and ultrasonication method | A substantial difference between the two groups treated with the commercially available oxiconazole nitrate cream and the matching created SLNs-loaded gel was found in the clinical investigation for the prepared oxiconazole nitrate SLNs gel. Over and beyond the commercial product, the produced gel showed superior clinical improvement. It may be said that the recently created oxiconazole nitrate sublingual tablets (SLNs) is a promising medication for the treatment of Tinea fungal infection. | (207) |
| 43. | Ethosomes | 99.5 nm | Cold method | The increased entrapment efficiency and superior stability profile of ethosomes were shown by the investigation to be extremely promising carriers for the transdermal distribution of oxiconazole nitrate. Finally, it was determined that the ethosomal gels loaded with oxyconazole nitrate were effectively developed and provide benefits of quick drug onset and maximal release with minimal adverse effects. | (208) | |
| 44. | Nanostructured lipid carriers | 172.8 nm | Hot emulsification followed by ultrasonication technique | Over a three-month period, the NLCs-incorporated gel remained stable and exhibited no change in its physical characteristics. The findings of the current research demonstrated that luliconazole topical gel loaded with NLCs is a potential topical formulation to treat dermatophytosis since there is not a gel version of luliconazole on the market at the moment. | (209) | |
| 45. | Luliconazole | Luliconazole-loaded nanostructured lipid carriers (NLCs) | 86.480 nm | Hot emulsification followed by ultrasonication technique | The current research effectively attests to the methodical QbD-based creation and optimization of NLCs formulation, demonstrating regulated luliconazole release, improved penetration, and better skin retention with a large dosage decrease that will assist to lessen the unwanted side effects. | (210) |
| 46. | Nanoemulgel (NEG) | 17 nm | The self-nanoemulsifying technique | When administered topically to the test animal, the produced product was confirmed to be secure. Erythema was not seen in the animals who received LUL-NEG. LUL-NEG might be a useful strategy for the safe and more effective localized distribution of LUL. | (211) |
7.1. Colloidal Carriers
7.1.1. Microemulsions
Microemulsions are colloidal dispersions that are transparent and thermodynamically stable. The incorporation of oils and surfactants in the formulation of a drug can enhance its ability to penetrate across the stratum corneum, thereby improving its solubility. Additionally, the ease of preparation of such a formulation is noteworthy. The aforementioned characteristics render it a desirable vehicle for the administration of pharmaceuticals through topical and transdermal routes. According to El Hadidy et al.’s findings, the application of voriconazole in the form of a microemulsion resulted in improved skin permeation, with a duration of up to four h when applied to pig skin. Additionally, this form of application demonstrated enhanced antimycotic activity against C. albicans.146 Patel et al. conducted a study in which they prepared an oil in water microemulsion system of ketoconazole. Their findings suggest that the ketoconazole exhibited enhanced percutaneous absorption and superior antifungal activity against C. albicans, a model fungus, in comparison to its conventional formulation.147 Comparable investigations were conducted on topical microemulsion formulations containing itraconazole, fluconazole, and clotrimazole. The findings indicated superior drug permeation, elevated skin retention, as well as enhanced effectiveness and tolerability.32 Projan et al. conducted a study in which they formulated microemulsions (MEs) containing itraconazole (ITZ). The system that was prepared demonstrated superior inhibitory properties against C. albicans and T. rubrum in comparison to a gel that is commercially available.148
7.1.2. Micelles
Micelles are submicroscopic vesicles characterized by a hydrophilic outer layer and a hydrophobic inner core. The structural configuration of this system renders it a desirable vehicle for administering hydrophobic pharmaceutical agents. The enhancement of effectiveness and selectivity can be achieved through the utilization of block copolymers that are sensitive to pH, temperature, ultrasound, or light, or via the conjugation of ligands for targeted delivery.149
Bachav et al. formulated aqueous micelle solutions of azole antifungal agents including fluconazole, econazole nitrate, and clotrimazole. They found that econazole showed better porcine skin deposition when compared with its commercial liposomal formulation.150
7.1.3. Submicron Emulsions or Nanoemulsions
These colloidal systems have been used to improve the penetrability, tolerability, and efficacy of antifungal medications used topically. Nystatin was developed as a nanoemulsion topical administration technology to lessen or completely eliminate side effects and systemic absorption. Ex vivo experiments on human skin revealed that there was no systemic absorption and that the amount of drug maintained was sufficient to provide antifungal activity. To comprehend the connection between skin penetration and a charge on the emulsion, numerous studies were conducted.151 In one of the investigations, it was discovered that positively charged miconazole nitrate microemulsions displayed better skin accumulation (almost twice as much) than their negatively charged counterparts.152 The authors Yang et al. formulated a delivery system using oleic acid-based self-micro emulsifying technology to encapsulate Clotrimazole. The systems that were prepared demonstrated antifungal properties against both planktonic and biofilm cells of C. albicans. Furthermore, the oleic acid-based self-microemulsifying delivery system (OA-SMEDDS) underwent an additional conversion to gel form. The gel that was prepared exhibited significant antifungal effectiveness against both wild-type and drug-resistant strains of C. albicans and C. tropicalis.153
7.2. Vesicular Carriers
7.2.1. Liposomes
Liposomes are vesicular structures composed of concentric bilayers wherein the hydrophilic core is enclosed by a phospholipid bilayer. Due to their ability to modify the biodistribution profile of a pharmaceutical agent, they are regarded as a promising mechanism for transdermal drug delivery.
The first commercially available liposomal formulation was AmBiosome R, a vesicular version of the antibiotic Amphotericin B (AmB). In 1990, Nexatar Company USA produced it. Following this, numerous new lipid-based innovative amphotericin-B delivery methods were created, and these formulations revealed a startling decrease in amphotericin-B-related side effects, such as a decrease in nephrotoxicity, while maintaining broad-spectrum antifungal effectiveness. These encouraging outcomes prompted researchers to create more cutting-edge antifungal formulations in order to improve the safety and effectiveness. However, all of these formulations were given parenthetically and were intended for systemic use. Around the world, numerous topical liposomal formulations have been developed to treat superficial mycotic infections.154 Agarwal and Katare developed liposomes containing miconazole nitrate and conducted a comparative analysis between the liposomal preparation and conventional cream formulations. The study revealed that the systems that were developed exhibited enhanced stability, superior permeability, and favorable size distribution. The researchers arrived at the conclusion that liposomes comprising of 97.3% saturated phosphatidylcholine content exhibited superior retention in comparison to liposomes formulated with 98.0% unsaturated phosphatidylcholine content.155 Guo et al. have reported that the combined application of ethanol and the anionic edge activator sodium dodecyl sulfate facilitates the targeted delivery of medicine to the skin strata, including the subcutaneous and deeper skin layers, resulting in the formation of small drug reservoirs. The utilization of lipid-based nanocarrier DEL (deformable liposomes) presents significant potential for the targeted and localized delivery and storage of lipophilic medication KTZ (Ketoconazole) in specific areas. In order to gain a deeper comprehension of the mechanisms underlying the significant enhancement of skin penetration and deposition by DEL, further investigation is required.156
In addition, Patel et al. developed ketoconazole liposomes loaded in a Carbopol gel. These liposomes were then compared with a simple gel and plain medication cream. The findings demonstrated that liposomal gel has a higher potential for the retention of drugs.157
7.2.2. Niosomes
Nonionic surfactant-based niosomes are a distinct class of liposomes. This approach surmounts the constraints commonly associated with traditional liposomes, offering a range of benefits, such as enhanced chemical stability, biocompatibility, biodegradability, reduced toxicity, ease of storage, and cost-effectiveness. The clinical efficacy of griseofulvin-loaded niosomes combined with Carbopol gel was formulated and investigated by Kassem et al. The researchers arrived at the conclusion that the niosomal preparation exhibited significantly higher rates of mycological cure, approximately 80%, in comparison to the liposomal formulation, which demonstrated a rate of approximately 50%.141 The authors, Firthouse et al., conducted a study in which they formulated and enhanced a niosomal gel containing miconazole. The gel was prepared using span 60, cholesterol, and sodium carboxymethyl cellulose as a gelling agent. The study revealed a significant release of the drug from the formulation, with a rate of 92.10% within a 24 h period.158
The researchers Shirshad et al. formulated a niosomal gel containing ketoconazole, utilizing a 1:0.2 ratio of Span 60 and cholesterol (CHO). The evaluation of the antifungal properties was conducted by using the cup-plate method. The outcomes of the zone of inhibition of the formulated preparation were compared with those of plain ketoconazole gel and commercially available ointment. The niosomes that were developed exhibited superior antifungal efficacy in comparison to the commercially available formulation. The enhanced antifungal potency of a gel formulation comprising ketoconazole in niosomal structure was evidenced to exhibit prolonged efficacy in contrast to formulations containing ketoconazole in non-niosomal configuration.159
The in vitro studies conducted by Gupta et al. investigated the niosomal formulation of fluconazole utilizing Span 40, Span 60, and Brij 72. The findings of the study indicated that the niosomes not only enhanced the cutaneous retention of the drug but also facilitated sustained drug release by creating depots in the skin.160
7.2.3. Ethosomes
Ethosomes can be characterized as lipid-based delivery systems that incorporate phospholipids and a significant concentration of alcohol. The research has revealed that the drug can be administered to deeper layers of the skin and has the potential to penetrate systemic circulation. The presence of alcohol in cosmetic formulations disrupts the lipid barrier of the skin, resulting in increased permeability.
Maheshwari and colleagues developed ethosomes and ultradeformable liposomes containing clotrimazole for the transdermal treatment of candidiasis. The findings indicate that the ethosomal formulation that was prepared exhibited a greater zone of inhibition when compared to both the deformable liposomal formulation and the commercially available formulation.161
Vermaand and Pathak developed a formulation of econazole-nitrate-loaded ethosomes and conducted a comparative analysis with hydroethanolic and liposomal gels. The percentage increase in drug diffusion observed with ethosomes was nearly twice as high as that observed with liposomal and hydroethanolic gels. The authors have demonstrated that the ethosomal gel exhibited remarkable antifungal efficacy, optimal storage stability, and controlled drug release.162
Luliconazole-loaded transethosomes (LCZ-TE) were prepared by El-Sonbaty et al. through utilization of ethanol injection and thin film hydration techniques. The nanovesicles that were produced underwent characterization to determine their size, zeta potential, entrapment efficiency, and in vitro drug release. Additionally, the ex vivo permeation and deposition of skin through rat skin were evaluated and compared with those of LCZ-solution in propylene glycol. As per the in vitro characterization of LCZ-TE, the ethanol injection technique yielded an average vesicle size of 246.3 ± 0.56 nm, while the thin-film method resulted in a size of 62.75 ± 0.16 nm. The thin film hydration method resulted in a higher percentage of LCZ deposition (54.79 ± 5.23%) compared to LCZ solution in propylene glycol (PG) (25.26 ± 2.84%) and ethanol injection (35.65 ± 4.354%), with a more than 2-fold increase in the former and a 1.5-fold increase in the latter. The findings of this study demonstrated the efficacy of the synthesized nanovesicles in addressing cutaneous fungal infections, thereby highlighting their therapeutic potential.163
Bhalaria et al. conducted a study on the clinical efficacy of fluconazole in ethosomal preparation against candida species. The findings of the study indicate that the antimycotic activity of fluconazole-loaded ethosomes was superior to that of both the marketed hydroethanolic solution and liposomal formulation of the drug.164
7.2.4. Transferosomes
Transferosomes are highly deformable liposomes mainly composed of surfactants and phospholipids. Along with acting as a carrier for transdermal or topical delivery of a drug, they can deliver the vaccine and genetic material very efficiently. Singh Shalu and colleagues formulated a transfersomal gel containing ketoconazole using a Box-Behnken design with three factors and three levels. Subsequently, the optimized transfersomal formulation was subjected to evaluation for its potential antimicrobial activity against C. albicans, as well as for its effects on skin irritation. The findings indicate that the formulation exhibited no observable signs of skin irritation. The formulation exhibited notable antimicrobial activity against C. albicans, as evidenced by its minimum inhibitory concentration (MIC) range of 4.57 to 4.6 μg/mL. The aforementioned results indicate that the use of transfersomal gel exhibits a high degree of promise for the delivery of ketoconazole.165 Abdellatif et al. conducted a study in which they formulated a transferosomal gel loaded with Sertaconazole (STZL). A comparative analysis was conducted to evaluate the antifungal efficacy of STZL-loaded transferosomal gel in contrast to a commercially available product, namely Dermofix. The study conducted in vivo demonstrated a significant prophylactic impact in the rat model with immune deficiency. The gel that was prepared exhibited a higher level of antifungal activity when compared to the formulation available in the market.166
7.3. Nanoparticulate Carriers
Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) are getting recognition as efficient carriers for topical delivery of a drug because of their high skin penetration capacity. The major benefit of these carriers is that they have a low toxicity. Their small size allows these lipid carriers to make close contact with skin layers.167
Terbinafine (TB)-loaded solid lipid nanoparticle (SLN) formulation was developed by Chen et al. as a potential strategy to improve permeability and decrease the frequency of dosing, thereby enhancing adherence and reducing adverse effects. The optimized formulation of TB’s SLN exhibits promising potential in augmenting the safety, cost-effectiveness, and tolerability of antifungal therapy. It was concluded that optimized SLN formulation of TB applied for 12 h might have efficacy comparable to that of Lamisil OnceTM for 24 h.168
Miconazole (MN)-SLN, or miconazole nitrate-loaded solid lipid nanoparticles, were created by Bhalekar et al. Ex-vivo tests were performed to determine whether a gel prepared with a specific MN-SLN dispersion could penetrate the skin of cadavers. The Franz diffusion cell was used to conduct the penetration study. The results show that the MN-SLN formulations showed a considerable increase in the cumulative uptake of MN in the skin when compared to a commercially available gel. These formulations also showed a noticeably enhanced capacity to target the skin. These findings imply that the tested MN-SLN formulation, which has skin targeting capabilities, has potential as a vehicle for the topical delivery of miconazole nitrate. Compared to conventional gel preparation, the developed miconazole nitrated encapsulated SLN dispersion improved skin targeting and drug accumulation in skin.169
Mukherjee et al. developed and assessed a system of Solid Lipid Nanoparticles (SLNs) containing itraconazole, with the aim of improving the drug’s therapeutic efficacy while reducing the required dosage. The formulated solid lipid nanoparticles (SLNs) present several benefits, such as a substantial drug-to-lipid ratio, efficient drug loading, reduced particle size and size distribution, and a moderate zeta potential of the particles. The SLNs that were prepared exhibit controlled release of itraconazole and demonstrate superior antifungal efficacy.170
Elshear et al. conducted a study to determine the antifungal activity of clotrimazole (Cz) and Thompson Seedless Vitis vinifera juice extract (VJ) loaded on chitosan nanoparticles (NCs). The NCs/VJ/Cz formulation was stable, with a substantial drug entrapment efficiency, i.e., 94.7%; polydispersity index (PDI) 0.24; zeta potential value +31; and an average size of 35.4 nm in diameter. Ex vivo and in vivo evaluations of the skin retention, penetration, and wound healing potentialities of NCs/VJ/Cz ointment were studied using experimental rats with injured skin fungal infections. The new antidermatophytic agent Cs/VJ/Cz ointment has a good wound healing capability and may be used to treat skin infections.171
7.4. Gelling Systems
Ghose et al. prepared terbinafine hydrochloride loaded polymeric nanosponge hydrogel. The antimicrobial potential of nanosponge hydrogel was evaluated against C. albicans and T. rubrum infections. The study’s results indicate that the hydrogel preparation exhibits nonirritating properties and possesses the capacity to impede the proliferation of fungal infections.172
The study conducted by Ozcan et al. involved an assessment of the efficacy of topically applied Terbinafine hydrochloride in hydrogel form, which resulted in greater drug release. The findings of the study suggest that the hydrogel formulation utilizing chitosan with the lowest molecular weight demonstrated the most substantial zone of inhibition in comparison to other chitosan-based gels and commercially available products.173
Sertaconazole microemulsion loaded hydrogel was prepared by Radwan et al. The hydrogel that was prepared and assessed for its antimycotic activity against C. albicans demonstrated larger zones of inhibition in comparison to the commercially available Dermofix cream.
8. Patents and Commercial Products for Dermatophytosis
There are numerous antifungal medications that are widely employed for the treatment of dermatophytosis and are currently available in the market. However, the existing literature on patents related to the diagnosis and delivery systems for superficial dermatophytosis is notably limited. Table 4 gives a detailed account on available product in market, patents related, and ongoing clinical trials to different topical drug delivery systems for infectious disorders in recent years.
Table 4. Available Patents, Commercial Products, and Ongoing Clinical Trials for the Treatment of Dermatophytosis.
| PRODUCTS | ||||
|---|---|---|---|---|
| S. No. | Active drug | Brand name | Manufacturing company | Approval year |
| 1. | Efinaconazole | Jublia | Valeant Pharmaceuticals | 2014 |
| 2. | Tavaborole | Kerydin | Pfizer | 2014 |
| 3. | Terbinafine | Lamisil AT | Dr. Reddy’s | |
| 4. | Miconazole | Vusion | Barrier Therapeutics, Inc. | 2006 |
| 5. | Ciclopirox | Penlac | Taro manufactures | 1999 |
| 6. | Nystatin | Nystop | Perrigo | 1996 |
| 7. | Ketoconazole | Adenosan | Pharmanik | 2000 |
| 8. | Akorazol | Collins | ||
| 9. | Kazinal | Asian Pharm | ||
| 10. | Kenalyn | Silom | ||
| 11. | Nizoral | Janssen-Cilag | ||
| 12. | Extina | Elegant india overseas | ||
| 13. | Xolegel | Aqua Pharmaceuticals | ||
| 14. | Econazole | Spectazole | Ortho-McNeil Pharmaceutical’s | 1982 |
| 15. | Naftifine | Naftin | Sebela International Limited | 2013 |
| 16. | Oxiconazole | Oxistat | Fougera Pharmaceuticals Inc. | 1988 |
| 17. | Nystatin | Nyamyc | Upsher-Smith Laboratories, LLC | 1971 |
| 18. | Ciclopirox | Loprox | Medimetriks Pharmaceuticals, Inc. · | 2004 |
| 19. | Terbinafine | Lamisil AT | Novartis Consumer Health, Inc. | 2007 |
| 20. | Miconazole | Secura | Smith and Nephew | 2010 |
| 21. | NuZole | Sun Pharmaceutical Industries Ltd. | ||
| 22. | Micaderm | ALTAIRE. | ||
| 23. | M-Zole 3 | Alpharma | ||
| 24. | Luliconazole | LUZU | Sun Pharmaceutical Industries Ltd. | 2013 |
| 25. | Clotrimazole | Mycelex | Ortho-McNeil Pharmaceutical, Inc. | 2004 |
| 26. | Eberconazole | Ebernet Cream | Dr. Reddy Laboratory | 2015 |
| 27. | Ebertif Cream | A. S Lifesciences | ||
| 28. | Ebzole cream | Will impex pharmachem Pvt. Ltd. | ||
| 29. | Eberclin | Canixa life sciences Pvt. Ltd. | ||
| 30. | Ebernet Lotion | Dr. Reddy Laboratory | ||
| 31. | Ebspor | Intas pharmaceuticals Ltd. | ||
| 32. | Eberfine cream | KLM Laboratories Pvt. Ltd. | ||
| 33. | Eberwin cream | Sun pharmaceuticals industries | ||
| 34. | Crurix | Curation healthcare Pvt. Ltd. | ||
| 35. | Ebermac | Macleods pharmaceutical Pvt. Ltd. | ||
| 36. | Eberjen | Jenburkt pharmaceuticals Ltd. | ||
| 37. | Eberfun cream | IPCA laboratories | ||
| 38. | Eberderm cream | Palsons Derma Pvt. Ltd. | ||
| 39. | Ebertif Cream | A. S Lifesciences | ||
| 40 | Ebzole cream | Will impex pharmachem Pvt. Ltd. | ||
| PATENTS | ||||
|---|---|---|---|---|
| S. No. | Inventor | Patents No./Approval Year | Title | Ref |
| 1. | Maibach HI, Luo EC, Hsu TM. | US6846837B2 (2005) | Topical administration of basic antifungal compositions to treat fungal infections of the nails. | (212) |
| 2. | Baker JR, Flack MR, Ciotti SM, Sutcliffe JA. | US20090269380A1 (2012) | Methods of Treating Fungal, yeast and mold infections | (213) |
| 3. | Blank LB, Gettings RL, White WC. | US4865844A (1989) | Method of Treating Tinea pedis and related dermatophytic infections | (214) |
| 4. | Godefroi EF, Eijcken CAMV. | US3485917A (1969) | Composition and method for combating fungus with imidazole carboxylates | (215) |
| 5. | Brillowska-Dabrowska AH. | WO2006133701A2 (2010) | Pcr Diagnostics of dermatophytes and other pathogenic fungi | (216) |
| 6. | Pier AC. | US5277904A (1994) | Broad Spectrum Dermatophyte Vaccine | (217) |
| 7. | Gray NM, Woosley RL. | WO1994014447A1 (2000) | Methods and compositions of (−) ketoconazole for treating fungal, yeast and dermatophyte infections | (218) |
| 8. | Freeman A, Segal R, Dror Y. | US7825104B2 (2010) | Methods and compositions for treating fungal infections | (219) |
| 9. | Scoppettuolo L, Peterson M, Almarsson O, Remenar J. | US20070293674A1 (2007) | Novel saperconazole crystalline forms and related processes, pharmaceutical compositions and methods | (220) |
| 10. | Balkovec JM, Bartizal K, Locke JB, Ong V, Sandison T, Thye D, Perlin DS, James KD. | US20180256673A1 (2018) | Methods for treating fungal infections | (221) |
| 11. | Khanuja SPS, Chaturvedi P, Singh AK, Shasany AK, Agarwal VK, Gupta VK, Gupta SC, Tripathy AK, Pal A, Saikia D. | US7291349B2 (2007) | Antidermatophytic preparation and use thereof | (222) |
| 12. | Trimble JO. | US8333981B2 (2012) | Antifungal treatment of nails | (223) |
| 13. | Genberg C, Beus CS, Savage PB. | US10238665B2 (2019) | Methods for treating fungal infections | (224) |
| 14. | Bartizal K, Daruwala P. | US20190374601A1 (2019) | Methods for treating fungal infections | (225) |
| 15. | Zhou B. | US20120310307A1 (2012) | Treatment of fungal infection by light irradiation | (226) |
| ONGOING
CLINICAL TRIALS | |||
|---|---|---|---|
| S. No. | Clinical trial no. | Sponsor | Title |
| 16. | NCT05777525 | University of Extremadura, Badajoz, Spain | Use of essential oils as natural therapies |
| 17. | NCT05363449 | Therapeutics, Inc., San Diego, CA, United States | Safety, tolerability, and pharmacokinetics of UHE-103 cream in Subjects with tinea cruris and/or tinea pedis |
| 18. | NCT05770245 | Dove Medical Press Ltd., Macclesfield, United Kingdom | Novel electrolyzed water spray treatment mild dermatophytosis |
9. Challenges Associated with Nanocarriers
The utilization of nanocarrier systems has demonstrated remarkable efficacy in the treatment of cutaneous fungal infections through the targeted delivery of bioactive agents to specific skin layers and affected regions. Vesicular nanocarriers exhibit significant utility owing to their ability to negotiate the skin via strategies such as fusion, absorption, and lipid exchange.227 Excessive skin penetration may pose a challenge, as it can result in the entry of drug molecules into the bloodstream, which is not conducive to the localized treatment of skin fungal infections. Hence, it is extremely important for pharmaceutical scientists to tackle these issues. Furthermore, it is imperative to address various obstacles such as ensuring safety, achieving clinical efficacy, implementing effective scaling up techniques, and determining the fate of vesicular nanocarriers in transdermal therapeutics.228 Further investigation is required to establish an optimal framework for a vesicular nanocarrier that can facilitate efficacious transdermal administration of antifungal therapeutics. Table 5 displays additional significant scientific obstacles linked to nanocarriers.
Table 5. Challenges Associated with Nanoformulations.
| S. No. | Challenges | Reason | Ref |
|---|---|---|---|
| 1. | Surface area and shape | The process of translocation to target cells has been augmented, resulting in the induction of endocytosis. | (228) |
| 2. | Aggregation | Initiation of cellular apoptosis. | (229) |
| 3. | Antigenicity | Immune response. | (230) |
| 4. | Surface charge | Initiation of opsonization. | (231) |
| 5. | Development of testing and analytical procedure | Outcome prediction is difficult. | (232) |
| 6. | Aspect ratio | Aggregation of nanocarrier results toxicity. | (233) |
10. Expert Opinion
Dermatophytosis poses a significant threat and is on the verge of becoming an epidemic. It is crucial to ensure proper treatment for this condition. Several therapeutic approaches have demonstrated elevated rates of efficacy in clinical contexts.234 Nonetheless, there are certain limitations associated with their usage, such as the emergence of antifungal resistance and the manifestation of adverse reactions.235 At present, dermatophyte infections are treated with drugs that have been approved by the FDA. Topical formulations of these medications, including creams, gels, lotions, and solutions, are readily accessible.236 Examples of these medications include naftifine 1%, butenafine 1%, clotrimazole 1%, econazole 1%, ketoconazole 1% and 2%, oxiconazole 1%, sulconazole 1%, ciclopirox 1%, and tolnaftate 1%. Regrettably, the topical application of these medications may elicit adverse reactions, including pruritus, erythema, a burning sensation, and cutaneous inflammation.237 In some cases, they may also lead to mild to severe gastrointestinal symptoms, abnormalities in liver function, taste loss, and headaches. Applying the medication topically on a regular basis is an efficient way to cure the infection and reach therapeutic levels in the tissue, but it can be tiresome and may cause the aforementioned adverse effects.238 In addition, various obstacles and problems must be addressed before the medicine can reach the desired tissue. Lipophilic (BCS Class II) antifungal medicines predominate, yet their larger molecular size might often limit their inherent penetrability. Therefore, nanoformulations offer a superior option for the topical formulation development of these medicines. These delivery systems can improve the bioavailability of these antifungals by delivering them in an optimal manner. Increased permeability and decreased dosage frequency and side effects can lead to better patient compliance with treatment.
In recent times, multiple efforts have been recorded to integrate diverse antifungal agents into innovative carriers, encompassing liposomes, solid lipid nanoparticles, nanostructured lipid carriers, ethosomes, transfersomes, and niosomes, with the aim of surmounting the constraints of the traditional treatment approaches for dermatophytosis due to their attributes, such as diminutive size, biocompatibility, and multifaceted nature. Certain nanocarriers were recently assessed through experiments on animal models. By incorporating antifungal agents like ciclopirox olamine and econazole into liposomes and testing them on guinea pig models of tinea, researchers found that these formulations were more effective as compared to conventional treatments.239 However, when griseofulvin was encapsulated in transfersomes, it successfully cured guinea pig models of dermatophytosis. Similarly, griseofulvin-loaded solid lipid nanoparticles were tested against Microsporum canis in guinea pigs and showed promising results.190
On the basis of these findings, clinical trials are required to investigate their application. One of these studies has progressed to clinical trials. Griseofulvin was formulated as liposomes and niosomes, for which clinical trials were conducted on 16 patients with tinea circinata. After 2.5 weeks of treatment, the maximum clinical and mycological cure rates were observed with niosomal preparations. Large-scale comparative studies should be conducted so that these novel carriers can be utilized to treat dermatophytosis in the general population.187
The primary objective is to develop a safe and efficacious therapy for the treatment of dermatophytosis. Appropriate delivery systems cannot be developed without a comprehensive understanding of the structure of the skin, the pathophysiology of the disease, and a well-established protocol for randomized, controlled clinical trials to support the commercialization of nanomedicines for the benefit of patients. Nanotechnology utilizing vesicular systems such as liposomes, transfersomes, and niosomes exhibits promising potential for further exploration of research on the market. According to clinical trials, liposome and niosome-based formulations have shown significant potential for the treatment of dermatophytosis. The key reason, proposed herein in the review article, appears to be quite convincing, as it suggests that the utilization of liposomal technology to encapsulate antifungal drugs can lead to beneficial effects across multiple dimensions. These achievements are anticipated to result in significant advantages, including reduced drug dosage requirements and improved effectiveness, as well as enhanced biosafety. We firmly believe that the technology developed, which is characterized by its ease-of-scalability, will effectively address the urgent needs of patients who are experiencing suffering. When considering all of these factors collectively, there is a notable disparity in comparison to the traditional approach to formulation.
11. Conclusion and Prospective
Dermatophytosis is a condition that affects both people and animals and is believed to be a widespread skin disease across the globe. Dermatophyte antifungal resistance is one of the most significant challenges and problems in the treatment of dermatophytis for both clinicians and researchers. Other significant issues include the limited number and availability of antifungal agents, the high prevalence rate and recent revolutions of dermatophytes, and the extended duration of treatment. Several therapeutic strategies are available for the treatment of dermatophytosis. Despite the fact that these interventions yield elevated clinical remission rates, they possess certain limitations. These drawbacks include resistance to antifungal medications, as well as the unpleasant effects that are associated with them. In recent years, efforts have been documented to incorporate various antifungal agents into novel carriers in order to offer better therapeutic action and overcome the limitations of conventional treatment strategies for dermatophytosis. The encapsulation of antifungal drugs within various carriers of nano- and micrometer-sized particles makes it possible to improve the treatment, particularly therapeutic activity and prolonged effect, while also allowing for triggered release by particular chemical and/or pathophysiologic stimuli. In addition, the utilization of such systems makes it possible to preserve the localized therapeutic effect and offers an enhancement of the drug’s accumulation in the skin. One can improve the therapeutic effectiveness of antifungal drugs delivered transdermally by increasing the efficiency of the delivery method. The objective of these endeavors is to enhance therapeutic alternatives for individuals afflicted with dermatophytosis. The goal of creating formulations with higher antifungal activity, extended retention of medication, better effectiveness, greater skin penetration of the drug, and sustained release of drug has been successfully accomplished by using nanocarriers.
Acknowledgments
The authors are grateful to the Department of Microbiology, Maharishi Markandeshwar Institute of Medical Sciences and Research (MMIMSR), Mullana for all the support and for providing all the necessary facilities. Waleed H. Almalki: The authors extend their appreciation to the Deanship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number: IFP22UQU4310387DSR180.
The authors declare no competing financial interest.
References
- White T. C.; Oliver B. G.; Gräser Y.; Henn M. R. Generating and Testing Molecular Hypotheses in the Dermatophytes. Eukaryot. Cell 2008, 7, 1238–1245. 10.1128/EC.00100-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar Nigam P. Antifungal Drugs and Resistance: Current Concepts. Our Dermatology Online 2015, 6, 212. 10.7241/ourd.20152.58. [DOI] [Google Scholar]
- Achterman R. R.; Smith A. R.; Oliver B. G.; White T. C. Sequenced Dermatophyte Strains: Growth Rate, Conidiation, Drug Susceptibilities, and Virulence in an Invertebrate Model. Fungal Genet. Biol. 2011, 48, 335–341. 10.1016/j.fgb.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T. C.; Findley K.; Dawson T. L.; Scheynius A.; Boekhout T.; Cuomo C. A.; Xu J.; Saunders C. W. Fungi on the Skin: Dermatophytes and Malassezia. Cold spring Harb. Perspect. Med. 2014, 4, a019802. 10.1101/cshperspect.a019802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltazar L. M.; Ray A.; Santos D. A.; Cisalpino P. S.; Friedman A. J.; Nosanchuk J. D. Antimicrobial Photodynamic Therapy: An Effective Alternative Approach to Control Fungal Infections. Front. Microbiol. 2015, 6, 202. 10.3389/fmicb.2015.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan M.; Inamadar A.; Mittal A.; Miskeen A. K.; Srinivas C. R.; Sardana K.; Godse K.; Patel K.; Rengasamy M.; Rudramurthy S.; Dogra S. Expert Consensus on the Management of Dermatophytosis in India (ECTODERM India). BMC Dermatol. 2018, 18, 1–11. 10.1186/s12895-018-0073-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan P.; Liu W. The Changing Face of Dermatophytic Infections Worldwide. Mycopathologia 2017, 182, 77–86. 10.1007/s11046-016-0082-8. [DOI] [PubMed] [Google Scholar]
- Philpot C. M. Geographical Distribution of the Dermatophytes: A Review. Epidemiol. Infect. 1978, 80, 301–313. 10.1017/S0022172400053663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirmirani P.; Tucker L.-Y. Epidemiologic Trends in Pediatric Tinea Capitis: A Population-Based Study from Kaiser Permanente Northern California. J. Am. Acad. Dermatol. 2013, 69, 916–921. 10.1016/j.jaad.2013.08.031. [DOI] [PubMed] [Google Scholar]
- Zhan P.; Geng C.; Li Z.; Jin Y.; Jiang Q.; Tao L.; Luo Y.; Xiong Z.; Wu S.; Li D.; et al. Evolution of Tinea Capitis in the Nanchang Area, Southern China: A 50-year Survey (1965–2014). Mycoses 2015, 58, 261–266. 10.1111/myc.12307. [DOI] [PubMed] [Google Scholar]
- Zhan P.; Li D.; Wang C.; Sun J.; Geng C.; Xiong Z.; Seyedmousavi S.; Liu W.; de Hoog G. S. Epidemiological Changes in Tinea Capitis over the Sixty Years of Economic Growth in China. Med. Mycol. 2015, 53, 691–698. 10.1093/mmy/myv057. [DOI] [PubMed] [Google Scholar]
- Mapelli E. T. M.; Cerri A.; Bombonato C.; Menni S. Tinea Capitis in the Paediatric Population in Milan, Italy: The Emergence of Trichophyton Violaceum. Mycopathologia 2013, 176, 243–246. 10.1007/s11046-013-9637-0. [DOI] [PubMed] [Google Scholar]
- Zhu M.; Li L.; Wang J.; Zhang C.; Kang K.; Zhang Q. Tinea Capitis in Southeastern China: A 16-Year Survey. Mycopathologia 2010, 169, 235–239. 10.1007/s11046-009-9260-2. [DOI] [PubMed] [Google Scholar]
- Durán-Valle M. T.; Regodón-Domínguez M.; Velasco-Rodríguez M. J.; Aragón A.; Gómez-Garcés J. L. Epidemia de Tiña Por Trichophyton Tonsurans En Un Área Sanitaria de La Comunidad de Madrid (España). Rev. Iberoam. Micol. 2016, 33, 126–128. 10.1016/j.riam.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Kelly B. P. Superficial Fungal Infections. Pediatr. Rev. 2012, 33, e22 10.1542/pir.33.4.e22. [DOI] [PubMed] [Google Scholar]
- Degreef H. J.; DeDoncker P. R. G. Current Therapy of Dermatophytosis. J. Am. Acad. Dermatol. 1994, 31, S25–S30. 10.1016/S0190-9622(08)81263-7. [DOI] [PubMed] [Google Scholar]
- Tainwala R.; Sharma Y. K. Pathogenesis of Dermatophytoses. Indian J. Dermatol. 2011, 56, 259. 10.4103/0019-5154.82476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermout S.; Tabart J.; Baldo A.; Mathy A.; Losson B.; Mignon B. Pathogenesis of Dermatophytosis. Mycopathologia 2008, 166, 267–275. 10.1007/s11046-008-9104-5. [DOI] [PubMed] [Google Scholar]
- Aljabre S. H. M.; Richardson M. D.; Scott E. M.; Rashid A.; Shankland G. S. Adherence of Arthroconidia and Germlings of Anthropophilic and Zoophilic Varieties of Trichophyton Mentagrophytes to Human Corneocytes as an Early Event in the Pathogenesis of Dermatophytosis. Clin. Exp. Dermatol. 1993, 18, 231–235. 10.1111/j.1365-2230.1993.tb02176.x. [DOI] [PubMed] [Google Scholar]
- Odom R. Pathophysiology of Dermatophyte Infections. J. Am. Acad. Dermatol. 1993, 28, S2–S7. 10.1016/S0190-9622(09)80300-9. [DOI] [PubMed] [Google Scholar]
- Reddy K. R.Fungal Infections (Mycoses): Dermatophytoses (Tinea, Ringworm). J. Gandaki Med. Coll. 2017, 10. 10.3126/jgmcn.v10i1.17901 [DOI] [Google Scholar]
- Ward H.; Parkes N.; Smith C.; Kluzek S.; Pearson R. Consensus for the Treatment of Tinea Pedis: A Systematic Review of Randomised Controlled Trials. J. Fungi 2022, 8, 351. 10.3390/jof8040351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo A. K.; Mahajan R. Management of Tinea Corporis, Tinea Cruris, and Tinea Pedis: A Comprehensive Review. Indian Dermatol. Online J. 2016, 7, 77. 10.4103/2229-5178.178099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oke O. O.; Onayemi O.; Olasode O. A.; Omisore A. G.; Oninla O. A. The Prevalence and Pattern of Superficial Fungal Infections among School Children in Ile-Ife, South-Western Nigeria. Dermatol. Res. Pract. 2014, 2014, 1. 10.1155/2014/842917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dei-Cas I.; Carrizo D.; Giri M.; Boyne G.; Domínguez N.; Novello V.; Acuña K.; Dei-Cas P. Infectious Skin Disorders Encountered in a Pediatric Emergency Department of a Tertiary Care Hospital in Argentina: A Descriptive Study. Int. J. Dermatol. 2019, 58, 288–295. 10.1111/ijd.14234. [DOI] [PubMed] [Google Scholar]
- Wahbah H. R.; Atallah R. B.; Eldahshan R. M.; Elsaie M. L. A Prospective Clinical and Trichoscopic Study of Tinea Capitis in Children during Treatment. Dermatol. Ther. 2022, 35, e15582 10.1111/dth.15582. [DOI] [PubMed] [Google Scholar]
- Gungor S.; Erdal M. S.; Aksu B. New Formulation Strategies in Topical Antifungal Therapy. J. Cosmetics Dermatological Sci. Appl. 2013, 3, 1A. 10.4236/jcdsa.2013.31A009. [DOI] [Google Scholar]
- Amichai B.; Grunwald M. H. Adverse Drug Reactions of the New Oral Antifungal Agents-Terbinafine, Fluconazole, and Itraconazole. Int. J. Dermatol. 1998, 37, 410–415. 10.1046/j.1365-4362.1998.00496.x. [DOI] [PubMed] [Google Scholar]
- Goldstein A. O.; Smith K. M.; Ives T. J.; Goldstein B. Mycotic Infections. Effective Management of Conditions Involving the Skin, Hair, and Nails. Geriatr. (Basel, Switzerland) 2000, 55, 40–42. [PubMed] [Google Scholar]
- Kumar L.; Verma S.; Bhardwaj A.; Vaidya S.; Vaidya B. Eradication of Superficial Fungal Infections by Conventional and Novel Approaches: A Comprehensive Review. Artif. cells, nanomedicine, Biotechnol. 2014, 42, 32–46. 10.3109/21691401.2013.769446. [DOI] [PubMed] [Google Scholar]
- Firooz A.; Nafisi S.; Maibach H. I. Novel Drug Delivery Strategies for Improving Econazole Antifungal Action. Int. J. Pharm. 2015, 495, 599–607. 10.1016/j.ijpharm.2015.09.015. [DOI] [PubMed] [Google Scholar]
- Kaur I. P.; Kakkar S. Topical Delivery of Antifungal Agents. Expert Opin. Drug Delivery 2010, 7, 1303–1327. 10.1517/17425247.2010.525230. [DOI] [PubMed] [Google Scholar]
- Georgopapadakou N. H.; Walsh T. J. Antifungal Agents: Chemotherapeutic Targets and Immunologic Strategies. Antimicrob. Agents Chemother. 1996, 40, 279–291. 10.1128/AAC.40.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameen M. Epidemiology of Superficial Fungal Infections. Clin. Dermatol. 2010, 28, 197–201. 10.1016/j.clindermatol.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Agarwal U.; Saran J.; Agarwal P. Clinico-Mycological Study of Dermatophytes in a Tertiary Care Centre in Northwest India. Indian J. Dermatol. Venereol. Leprol. 2014, 80, 194. 10.4103/0378-6323.129434. [DOI] [PubMed] [Google Scholar]
- Jartarkar S. R.; Patil A.; Goldust Y.; Cockerell C. J.; Schwartz R. A.; Grabbe S.; Goldust M. Pathogenesis, Immunology and Management of Dermatophytosis. J. Fungi 2022, 8, 39. 10.3390/jof8010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmanan A.; Ganeshkumar P.; Mohan S. R.; Hemamalini M.; Madhavan R. Epidemiological and Clinical Pattern of Dermatomycoses in Rural India. Indian J. Med. Microbiol. 2015, 33, S134–S136. 10.4103/0255-0857.150922. [DOI] [PubMed] [Google Scholar]
- Zorab H. K.; Amin S. Q.; Mahmood H. J.; Mustafa H. H.; Abdulrahman N. M. A. Dermatophytosis. One Health Triad 2023, 3, 99–106. 10.47278/book.oht/2023.83. [DOI] [Google Scholar]
- Bhari R.; Kaur M.. Fungal Keratinases: Enzymes with Immense Biotechnological Potential. In Fungal Resources for Sustainable Economy: Current Status and Future Perspectives; Springer, 2023; pp 89–125. [Google Scholar]
- Mast N.; Zheng W.; Stout C. D.; Pikuleva I. A. Antifungal Azoles: Structural Insights into Undesired Tight Binding to Cholesterol-Metabolizing CYP46A1. Mol. Pharmacol. 2013, 84, 86–94. 10.1124/mol.113.085902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falahati M.; Akhlaghi L.; Lari A. R.; Alaghehbandan R. Epidemiology of Dermatophytoses in an Area South of Tehran, Iran. Mycopathologia 2003, 156, 279–287. 10.1023/B:MYCO.0000003560.65857.cf. [DOI] [PubMed] [Google Scholar]
- Hayette M.-P.; Sacheli R. Dermatophytosis, Trends in Epidemiology and Diagnostic Approach. Curr. Fungal Infect. Rep. 2015, 9, 164–179. 10.1007/s12281-015-0231-4. [DOI] [Google Scholar]
- Girmenia C. New Generation Azole Antifungals in Clinical Investigation. Expert Opin. Investig. Drugs 2009, 18, 1279–1295. 10.1517/13543780903176407. [DOI] [PubMed] [Google Scholar]
- Vanden Bossche H.; Marichal P.; Le Jeune L.; Coene M. C.; Gorrens J.; Cools W. Effects of Itraconazole on Cytochrome P-450-Dependent Sterol 14 Alpha-Demethylation and Reduction of 3-Ketosteroids in Cryptococcus Neoformans. Antimicrob. Agents Chemother. 1993, 37, 2101–2105. 10.1128/AAC.37.10.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman I.; Summerbell R. C. The Dermatophytes. Clin. Microbiol. Rev. 1995, 8, 240–259. 10.1128/CMR.8.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epidemiology and Management of Common Skin Diseases in Children in Developing Countries; World Health Organization, 2005. [Google Scholar]
- Hogewoning S. L.; van Hees C.; Naafs B.; van der Stek J.; Moser R. Skin Diseases among Children in Africa. Pediatr Dermatol 2005, 22, 6–10.15660888 [Google Scholar]
- Al-Janabi A. A. H. S. Dermatophytosis: Causes, Clinical Features, Signs and Treatment. J. Symptoms Signs 2014, 3, 200–203. [Google Scholar]
- Gupta A. K.; Stec N. Emerging Drugs for the Treatment of Onychomycosis. Expert Opin. Emerg. Drugs 2019, 24, 213–220. 10.1080/14728214.2019.1685493. [DOI] [PubMed] [Google Scholar]
- Barrett-Bee K.; Newboult L.; Pinder P. Biochemical Changes Associated with the Antifungal Action of the Triazole ICI 153,066 on Candida Albicans and Trichophyton Quinckeanum. FEMS Microbiol. Lett. 1991, 79, 127–132. 10.1111/j.1574-6968.1991.tb04517.x. [DOI] [PubMed] [Google Scholar]
- Jena A. K.; Lenka R. K.; Sahu M. C. Dermatophytosis in a Tertiary Care Teaching Hospital of Odisha: A Study of 100 Cases of Superficial Fungal Skin Infection. Indian J. Pub Heal. Res. Dev. 2018, 9, 7. 10.5958/0976-5506.2018.01416.X. [DOI] [Google Scholar]
- Gupta C. M.; Tripathi K.; Tiwari S.; Rathore Y.; Nema S.; Dhanvijay A. G. Current Trends of Clinicomycological Profile of Dermatophytosis in Central India. IOSR-JDMS 2014, 13, 23–26. 10.9790/0853-131032326. [DOI] [Google Scholar]
- Seebacher C.; Bouchara J.-P.; Mignon B. Updates on the Epidemiology of Dermatophyte Infections. Mycopathologia 2008, 166, 335–352. 10.1007/s11046-008-9100-9. [DOI] [PubMed] [Google Scholar]
- Nes W. D.; Janssen G. G.; Crumley F. G.; Kalinowska M.; Akihisa T. The Structural Requirements of Sterols for Membrane Function in Saccharomyces Cerevisiae. Arch. Biochem. Biophys. 1993, 300, 724–733. 10.1006/abbi.1993.1100. [DOI] [PubMed] [Google Scholar]
- Miranda S.; Billoir P.; Damian L.; Thiebaut P. A.; Schapman D.; Le Besnerais M.; Jouen F.; Galas L.; Levesque H.; Le Cam-Duchez V.; et al. Hydroxychloroquine Reverses the Prothrombotic State in a Mouse Model of Antiphospholipid Syndrome: Role of Reduced Inflammation and Endothelial Dysfunction. PLoS One 2019, 14, e0212614 10.1371/journal.pone.0212614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogra S.; Narang T. Emerging Atypical and Unusual Presentations of Dermatophytosis in India. Clin. Dermatology Rev. 2017, 1, 12. 10.4103/CDR.CDR_39_17. [DOI] [Google Scholar]
- Borah P.; Hazarika S.; Sharma D.; Venugopala K. N.; Chopra D.; Al-Shar’i N. A.; Hemalatha S.; Shakya A. K.; Acharya P. C.; Deb P. K.. Systemic and Topical Antifungal Drugs. In Medicinal Chemistry of Chemotherapeutic Agents; Elsevier, 2023; pp 285–315. [Google Scholar]
- Gadadavar S.; Shilpa H. S.; Patil C. S.; Vinay P. S.; Shettar N. Clinico-Mycological Study of Dermatophytosis at a Tertiary Care Hospital in Belagavi, Karnataka, India. Int. J. Curr. Microbiol Appl. Sci. 2018, 7, 1872–1880. 10.20546/ijcmas.2018.705.220. [DOI] [Google Scholar]
- Al-Khikani F. O. Major Factors Associated with Worldwide Dermatophytosis Predominance. MGM J. Med. Sci. 2020, 7, 232. 10.4103/mgmj.mgmj_64_20. [DOI] [Google Scholar]
- Havlickova B.; Czaika V. A.; Friedrich M. Epidemiological Trends in Skin Mycoses Worldwide. Mycoses 2008, 51, 2–15. 10.1111/j.1439-0507.2008.01606.x. [DOI] [PubMed] [Google Scholar]
- Ayatollahi A.; Firooz A.; Lotfali E.; Mojab F.; Fattahi M. Herbal Therapy for the Management of Seborrheic Dermatitis: A Narrative Review. Recent Adv. Anti-Infective Drug Discovery Former. Recent Patents Anti-Infective Drug Discovery 2021, 16, 209–226. 10.2174/2772434416666211029113213. [DOI] [PubMed] [Google Scholar]
- Loeffler J.; Stevens D. A. Antifungal Drug Resistance. Clin. Infect. Dis. 2003, 36, S31–S41. 10.1086/344658. [DOI] [PubMed] [Google Scholar]
- Van der Weerden N. L.; Bleackley M. R.; Anderson M. A. Properties and Mechanisms of Action of Naturally Occurring Antifungal Peptides. Cell. Mol. life Sci. 2013, 70, 3545–3570. 10.1007/s00018-013-1260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnie J. P.; Carter T. L.; Hodgetts S. J.; Matthews R. C. Fungal Heat-Shock Proteins in Human Disease. FEMS Microbiol. Rev. 2006, 30, 53–88. 10.1111/j.1574-6976.2005.00001.x. [DOI] [PubMed] [Google Scholar]
- Kathiravan M. K.; Salake A. B.; Chothe A. S.; Dudhe P. B.; Watode R. P.; Mukta M. S.; Gadhwe S. The Biology and Chemistry of Antifungal Agents: A Review. Bioorg. Med. Chem. 2012, 20, 5678–5698. 10.1016/j.bmc.2012.04.045. [DOI] [PubMed] [Google Scholar]
- Rostom S. A. F.; Ashour H. M. A.; Abd El Razik H. A.; Abd El Fattah H.; El-Din N. N. Azole Antimicrobial Pharmacophore-Based Tetrazoles: Synthesis and Biological Evaluation as Potential Antimicrobial and Anticonvulsant Agents. Bioorg. Med. Chem. 2009, 17, 2410–2422. 10.1016/j.bmc.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Spampinato C.; Leonardi D. Candida Infections, Causes, Targets, and Resistance Mechanisms: Traditional and Alternative Antifungal Agents. Biomed Res. Int. 2013, 2013, 1. 10.1155/2013/204237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tyle J. H. Ketoconazole; Mechanism of Action, Spectrum of Activity, Pharmacokinetics, Drug Interactions, Adverse Reactions and Therapeutic Use. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1984, 4, 343–373. 10.1002/j.1875-9114.1984.tb03398.x. [DOI] [PubMed] [Google Scholar]
- De Beule K.; Van Gestel J. Pharmacology of Itraconazole. Drugs 2001, 61, 27–37. 10.2165/00003495-200161001-00003. [DOI] [PubMed] [Google Scholar]
- Lipner S. R.; Scher R. K. Onychomycosis: Treatment and Prevention of Recurrence. J. Am. Acad. Dermatol. 2019, 80, 853–867. 10.1016/j.jaad.2018.05.1260. [DOI] [PubMed] [Google Scholar]
- Hitchcock C. A.; Dickinson K.; Brown S. B.; Evans E.G. V; Adams D. J. Interaction of Azole Antifungal Antibiotics with Cytochrome P-450-Dependent 14α-Sterol Demethylase Purified from Candida Albicans. Biochem. J. 1990, 266, 475–480. 10.1042/bj2660475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piérard G. E.; Hermanns-Lê T.; Delvenne P.; Piérard-Franchimont C. Miconazole, a Pharmacological Barrier to Skin Fungal Infections. Expert Opin. Pharmacother. 2012, 13, 1187–1194. 10.1517/14656566.2012.687047. [DOI] [PubMed] [Google Scholar]
- Stuehler C.; Khanna N.; Bozza S.; Zelante T.; Moretti S.; Kruhm M.; Lurati S.; Conrad B.; Worschech E.; Stevanović S.; et al. Cross-Protective TH1 Immunity against Aspergillus Fumigatus and Candida Albicans. Blood, J. Am. Soc. Hematol. 2011, 117, 5881–5891. 10.1182/blood-2010-12-325084. [DOI] [PubMed] [Google Scholar]
- Khanna N.; Stuehler C.; Conrad B.; Lurati S.; Krappmann S.; Einsele H.; Berges C.; Topp M. S. Generation of a Multipathogen-Specific T-Cell Product for Adoptive Immunotherapy Based on Activation-Dependent Expression of CD154. Blood, J. Am. Soc. Hematol. 2011, 118, 1121–1131. 10.1182/blood-2010-12-322610. [DOI] [PubMed] [Google Scholar]
- Kumaresan P. R.; Manuri P. R.; Albert N. D.; Maiti S.; Singh H.; Mi T.; Roszik J.; Rabinovich B.; Olivares S.; Krishnamurthy J.; et al. Bioengineering T Cells to Target Carbohydrate to Treat Opportunistic Fungal Infection. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 10660–10665. 10.1073/pnas.1312789111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaresan P. R.; Da Silva T. A.; Kontoyiannis D. P. Methods of Controlling Invasive Fungal Infections Using CD8+ T Cells. Front. Immunol. 2018, 8, 1939. 10.3389/fimmu.2017.01939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici N. P.; Del Poeta M. New Insights on the Development of Fungal Vaccines: From Immunity to Recent Challenges. Mem. Inst. Oswaldo Cruz 2015, 110, 966–973. 10.1590/0074-02760150335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal B. H.; Veys P.; Malech H.; Cowan M. J. Chronic Granulomatous Disease: Lessons from a Rare Disorder. Biol. Blood Marrow Transplant. 2011, 17, S123–S131. 10.1016/j.bbmt.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L. L.; Spurrell J. C. L.; Wang J. F.; Neely G. G.; Epelman S.; Krensky A. M.; Mody C. H. CD8 T Cell-Mediated Killing of Cryptococcus Neoformans Requires Granulysin and Is Dependent on CD4 T Cells and IL-15. J. Immunol. 2002, 169, 5787–5795. 10.4049/jimmunol.169.10.5787. [DOI] [PubMed] [Google Scholar]
- Nanjappa S. G.; Klein B. S. Vaccine Immunity against Fungal Infections. Curr. Opin. Immunol. 2014, 28, 27. 10.1016/j.coi.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis J. N.; Meintjes G.; Rebe K.; Williams G. N.; Bicanic T.; Williams A.; Schutz C.; Bekker L.-G.; Wood R.; Harrison T. S. Adjunctive Interferon-γ Immunotherapy for the Treatment of HIV-Associated Cryptococcal Meningitis: A Randomized Controlled Trial. AIDS 2012, 26, 1105. 10.1097/QAD.0b013e3283536a93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas P. G.; Bustamante B.; Ticona E.; Hamill R. J.; Johnson P. C.; Reboli A.; Aberg J.; Hasbun R.; Hsu H. H. Recombinant Interferon-Γlb as Adjunctive Therapy for AIDS-Related Acute Cryptococcal Meningitis. J. Infect. Dis. 2004, 189, 2185–2191. 10.1086/420829. [DOI] [PubMed] [Google Scholar]
- Dadachova E.; Nakouzi A.; Bryan R. A.; Casadevall A. Ionizing Radiation Delivered by Specific Antibody Is Therapeutic against a Fungal Infection. Proc. Natl. Acad. Sci. U. S. A. 2003, 100, 10942–10947. 10.1073/pnas.1731272100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen R. A.; Pappas P. G.; Perfect J.; Aberg J. A.; Casadevall A.; Cloud G. A.; James R.; Filler S.; Dismukes W. E. Phase I Evaluation of the Safety and Pharmacokinetics of Murine-Derived Anticryptococcal Antibody 18B7 in Subjects with Treated Cryptococcal Meningitis. Antimicrob. Agents Chemother. 2005, 49, 952–958. 10.1128/AAC.49.3.952-958.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braem S. G. E.; Rooijakkers S. H. M.; Van Kessel K. P. M.; De Cock H.; Wösten H. A. B.; Van Strijp J. A. G.; Haas P.-J. A. Effective Neutrophil Phagocytosis of Aspergillus Fumigatus Is Mediated by Classical Pathway Complement Activation. J. Innate Immun. 2015, 7, 364–374. 10.1159/000369493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safdar A.; Rodriguez G.; Ohmagari N.; Kontoyiannis D. P.; Rolston K. V.; Raad I. I.; Champlin R. E. The Safety of Interferon-γ-1b Therapy for Invasive Fungal Infections after Hematopoietic Stem Cell Transplantation. Cancer 2005, 103, 731–739. 10.1002/cncr.20883. [DOI] [PubMed] [Google Scholar]
- Ulrich S.; Ebel F. Monoclonal Antibodies as Tools to Combat Fungal Infections. J. Fungi 2020, 6, 22. 10.3390/jof6010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Effron G. Rezafungin—Mechanisms of Action, Susceptibility and Resistance: Similarities and Differences with the Other Echinocandins. J. fungi 2020, 6, 262. 10.3390/jof6040262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel J. D.; Nyirjesy P. Oteseconazole: An Advance in Treatment of Recurrent Vulvovaginal Candidiasis. Future Microbiol. 2021, 16, 1453–1461. 10.2217/fmb-2021-0173. [DOI] [PubMed] [Google Scholar]
- Jallow S.; Govender N. P. Ibrexafungerp: A First-in-Class Oral Triterpenoid Glucan Synthase Inhibitor. J. Fungi 2021, 7, 163. 10.3390/jof7030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirseren D. D. New Therapeutic Options in the Management of Superficial Fungal Diseases. Dermatol. Ther. 2020, 33, e12855 10.1111/dth.12855. [DOI] [PubMed] [Google Scholar]
- Wiederhold N. P. Review of the Novel Investigational Antifungal Olorofim. J. Fungi 2020, 6, 122. 10.3390/jof6030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox L. P.; Merk H. F.; Bickers D. R. Dermatological Pharmacology. Goodman Gilman’s Pharmacol. Basis Ther. 2006, 1679–1706. [Google Scholar]
- Gupta M.; Sharma V.; Chauhan N. S.. Promising Novel Nanopharmaceuticals for Improving Topical Antifungal Drug Delivery. In Nano-and Microscale Drug Delivery Systems; Elsevier, 2017; pp 197–228. [Google Scholar]
- Havlickova B.; Friedrich M. The Advantages of Topical Combination Therapy in the Treatment of Inflammatory Dermatomycoses. Mycoses 2008, 51, 16–26. 10.1111/j.1439-0507.2008.01615.x. [DOI] [PubMed] [Google Scholar]
- Öztürk A. A.; Yenilmez E. Pharmacological and Pharmaceutical Technological Overview for Seborrheic Dermatitis: A Review about Topical Application and New Approaches. ACTA Pharm. Sci. 2018, 56, 57–80. 10.23893/1307-2080.APS.05626. [DOI] [Google Scholar]
- Allegra S.; Fatiguso G.; De Francia S.; Favata F.; Pirro E.; Carcieri C.; De Nicolò A.; Cusato J.; Di Perri G.; D’Avolio A. Pharmacokinetic Evaluation of Oral Itraconazole for Antifungal Prophylaxis in Children. Clin. Exp. Pharmacol. Physiol. 2017, 44, 1083–1088. 10.1111/1440-1681.12822. [DOI] [PubMed] [Google Scholar]
- Cauwenbergh G.; Degreef H.; Heykants J.; Woestenborghs R.; Van Rooy P.; Haeverans K. Pharmacokinetic Profile of Orally Administered Itraconazole in Human Skin. J. Am. Acad. Dermatol. 1988, 18, 263–268. 10.1016/S0190-9622(88)70037-7. [DOI] [PubMed] [Google Scholar]
- Singh A.; Masih A.; Khurana A.; Singh P. K.; Gupta M.; Hagen F.; Meis J. F.; Chowdhary A. High Terbinafine Resistance in Trichophyton Interdigitale Isolates in Delhi, India Harbouring Mutations in the Squalene Epoxidase Gene. Mycoses 2018, 61, 477–484. 10.1111/myc.12772. [DOI] [PubMed] [Google Scholar]
- Ghannoum M. A.; Long L.; Kim H. G.; Cirino A. J.; Miller A. R.; Mallefet P. Efficacy of Terbinafine Compared to Lanoconazole and Luliconazole in the Topical Treatment of Dermatophytosis in a Guinea Pig Model. Med. Mycol. 2010, 48, 491–497. 10.3109/13693780903373811. [DOI] [PubMed] [Google Scholar]
- Aly R.; Bayles C. I.; Oakes R. A.; Bibel D. J.; Maibach H. I. Topical Griseofulvin in the Treatment of Dermatophytoses. Clin. Exp. Dermatol. 1994, 19, 43–46. 10.1111/j.1365-2230.1994.tb01113.x. [DOI] [PubMed] [Google Scholar]
- Vazquez J. A. Optimal Management of Oropharyngeal and Esophageal Candidiasis in Patients Living with HIV Infection. HIV/AIDS (Auckland, NZ) 2010, 2, 89. 10.2147/HIV.S6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu P. R.; Pravin A. J. S.; Deshmukh G.; Dhoot D.; Samant A.; Kotak B. Efficacy and Safety of Terbinafine 500 Mg Once Daily in Patients with Dermatophytosis. Indian J. Dermatol. 2017, 62, 395. 10.4103/ijd.IJD_191_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W. L.; Shah V. P.; Riegelman S. Griseofulvin Levels in Stratum Corneum: Study after Oral Administration in Man. Arch. Dermatol. 1972, 106, 344–348. 10.1001/archderm.1972.01620120032006. [DOI] [PubMed] [Google Scholar]
- Robertson M. H.; Rich P.; Parker F.; Hanifin J. M. Ketoconazole in Griseofulvin-Resistant Dermatophytosis. J. Am. Acad. Dermatol. 1982, 6, 224–229. 10.1016/S0190-9622(82)70015-5. [DOI] [PubMed] [Google Scholar]
- Haneke E. Retention of Ketoconazole in the Skin Following Oral Treatment. Hautarzt. 1987, 38, 93–96. [PubMed] [Google Scholar]
- Jones H. E. Problems of Resistant Dermatophytes. J. Am. Acad. Dermatol. 1990, 23, 779–781. 10.1016/0190-9622(90)70287-R. [DOI] [PubMed] [Google Scholar]
- Manzano-Gayosso P.; Mendez-Tovar L. J.; Hernandez-Hernandez F.; Lopez-Martinez R. Antifungal Resistance: An Emerging Problem in Mexico. Gac. Med. Mex. 2008, 144, 23–26. [PubMed] [Google Scholar]
- Gupta A. K.; Lyons D. C. A. The Rise and Fall of Oral Ketoconazole. J. Cutan. Med. Surg. 2015, 19, 352–357. 10.1177/1203475415574970. [DOI] [PubMed] [Google Scholar]
- Wildfeuer A.; Faergemann J.; Laufen H.; Pfaff G.; Zimmermann T.; Seidl H. P.; Lach P. Bioavailability of Fluconazole in the Skin after Oral Medication: Bioverfügbarkeit von Fluconazol in Der Haut Nach Oraler Medikation. Mycoses 1994, 37, 127–130. 10.1111/j.1439-0507.1994.tb00788.x. [DOI] [PubMed] [Google Scholar]
- Gupta A. K.; Cooper E. A. Update in Antifungal Therapy of Dermatophytosis. Mycopathologia 2008, 166, 353–367. 10.1007/s11046-008-9109-0. [DOI] [PubMed] [Google Scholar]
- Stary A.; Sarnow E. Fluconazole in the Treatment of Tinea Corporis and Tinea Cruris. Dermatology 1998, 196, 237–241. 10.1159/000017881. [DOI] [PubMed] [Google Scholar]
- Nozickova M.; Koudelkova V.; Kulikova Z.; Malina L.; Urbanowski S.; Silny W. A Comparison of the Efficacy of Oral Fluconazole, 150 Mg/Week versus 50 Mg/Day, in the Treatment of Tinea Corporis, Tinea Cruris, Tinea Pedis, and Cutaneous Candidosis. Int. J. Dermatol. 1998, 37, 703–705. 10.1046/j.1365-4362.1998.00541.x. [DOI] [PubMed] [Google Scholar]
- Suchil P.; Montero Gei F.; Robles M.; Perera-Ramirez A.; Welsh O.; Male O. Once-weekly Oral Doses of Fluconazole 150 Mg in the Treatment of Tinea Corporis/Cruris and Cutaneous Candidiasis. Clin. Exp. Dermatol. 1992, 17, 397–401. 10.1111/j.1365-2230.1992.tb00246.x. [DOI] [PubMed] [Google Scholar]
- Sharma P.; Bhalla M.; Thami G. P.; Chander J. Evaluation of Efficacy and Safety of Oral Terbinafine and Itraconazole Combination Therapy in the Management of Dermatophytosis. J. Dermatolog. Treat. 2020, 31, 749–753. 10.1080/09546634.2019.1612835. [DOI] [PubMed] [Google Scholar]
- Firoze S.; Khan H. M.; Fatima N. Evaluation of Antifungal Susceptibility Testing Methods for Dermatophytes. Inter. J. Dent. Med. Sci. Res. 2022, 4, 116–124. 10.35629/5252-0405116124. [DOI] [Google Scholar]
- Singh S.; Chandra U.; Verma P.; Anchan V. N.; Tilak R. Limited effectiveness of four oral antifungal drugs (fluconazole, griseofulvin, itraconazole and terbinafine) in the current epidemic of altered dermatophytosis in India: results of a randomized pragmatic trial. BJD 2020, 183, 840–846. 10.1111/bjd.19146. [DOI] [PubMed] [Google Scholar]
- Cavassin F. B.; Baú-Carneiro J. L.; Vilas-Boas R. R.; Queiroz-Telles F. Sixty Years of Amphotericin B: An Overview of the Main Antifungal Agent Used to Treat Invasive Fungal Infections. Infect. Dis. Ther. 2021, 10, 115–147. 10.1007/s40121-020-00382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regidor P. A.; Thamkhantho M.; Chayachinda C.; Palacios S. Miconazole for the Treatment of Vulvovaginal Candidiasis. In Vitro, in Vivo and Clinical Results. Review of the Literature. J. Obstet. Gynaecol. (Lahore). 2023, 43, 2195001. 10.1080/01443615.2023.2195001. [DOI] [PubMed] [Google Scholar]
- Hashemian S. M.; Farhadi T.; Velayati A. A. Caspofungin: A Review of Its Characteristics, Activity, and Use in Intensive Care Units. Expert Rev. Anti. Infect. Ther. 2020, 18, 1213–1220. 10.1080/14787210.2020.1794817. [DOI] [PubMed] [Google Scholar]
- Carmo A.; Rocha M.; Pereirinha P.; Tomé R.; Costa E. Antifungals: From Pharmacokinetics to Clinical Practice. Antibiotics 2023, 12, 884. 10.3390/antibiotics12050884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulkowski M. S.; Cooper C.; Hunyady B.; Jia J.; Ogurtsov P.; Peck-Radosavljevic M.; Shiffman M. L.; Yurdaydin C.; Dalgard O. Management of Adverse Effects of Peg-IFN and Ribavirin Therapy for Hepatitis C. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 212–223. 10.1038/nrgastro.2011.21. [DOI] [PubMed] [Google Scholar]
- Nagarwal R. C.; Kumar R.; Dhanawat M.; Das N.; Pandit J. K. Nanocrystal Technology in the Delivery of Poorly Soluble Drugs: An Overview. Curr. Drug Delivery 2011, 8, 398–406. 10.2174/156720111795767988. [DOI] [PubMed] [Google Scholar]
- Auvinen T.; Tiihonen R.; Soini M.; Wangel M.; Sipponen A.; Jokinen J. J. Efficacy of Topical Resin Lacquer, Amorolfine and Oral Terbinafine for Treating Toenail Onychomycosis: A Prospective, Randomized, Controlled, Investigator-blinded, Parallel-group Clinical Trial. Br. J. Dermatol. 2015, 173, 940–948. 10.1111/bjd.13934. [DOI] [PubMed] [Google Scholar]
- Yu Y. Q.; Yang X.; Wu X. F.; Fan Y. B. Enhancing Permeation of Drug Molecules across the Skin via Delivery in Nanocarriers: Novel Strategies for Effective Transdermal Applications. Front. Bioeng. Biotechnol. 2021, 9, 646554. 10.3389/fbioe.2021.646554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanglard D. Emerging Threats in Antifungal-Resistant Fungal Pathogens. Front. Med. 2016, 3, 11. 10.3389/fmed.2016.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres N.T. de A.; Maranhão F. C. A.; Rossi A.; Martinez-Rossi N. M. Dermatophytes: Host-Pathogen Interaction and Antifungal Resistance. An. Bras. Dermatol. 2010, 85, 657–667. 10.1590/S0365-05962010000500009. [DOI] [PubMed] [Google Scholar]
- Ghannoum M. A.; Chaturvedi V.; Espinel-Ingroff A.; Pfaller M. A.; Rinaldi M. G.; Lee-Yang W.; Warnock D. W. Intra-and Interlaboratory Study of a Method for Testing the Antifungal Susceptibilities of Dermatophytes. J. Clin. Microbiol. 2004, 42, 2977–2979. 10.1128/JCM.42.7.2977-2979.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeputte P.; Ferrari S.; Coste A. T. Antifungal Resistance and New Strategies to Control Fungal Infections. Int. J. Microbiol. 2012, 2012, 713687. 10.1155/2012/713687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi P.; Singh A.; Pundir S.; Parashar A.; Upadhyay N.; Agarwal S.; Chauhan R.; Tambuwala M. M. Essential Oil and Nanocarrier Based Formulations Approaches for Vaginal Candidiasis. Ther. Delivery 2023, 14, 207–225. 10.4155/tde-2022-0058. [DOI] [PubMed] [Google Scholar]
- Nagaraj S.; Manivannan S.; Narayan S. Potent Antifungal Agents and Use of Nanocarriers to Improve Delivery to the Infected Site: A Systematic Review. J. Basic Microbiol. 2021, 61, 849–873. 10.1002/jobm.202100204. [DOI] [PubMed] [Google Scholar]
- El Rabey H. A.; Almutairi F. M.; Alalawy A. I.; Al-Duais M. A.; Sakran M. I.; Zidan N. S.; Tayel A. A. Augmented Control of Drug-Resistant Candida Spp. via Fluconazole Loading into Fungal Chitosan Nanoparticles. Int. J. Biol. Macromol. 2019, 141, 511–516. 10.1016/j.ijbiomac.2019.09.036. [DOI] [PubMed] [Google Scholar]
- Kelidari H. R.; Babaei R.; Nabili M.; Shokohi T.; Saeedi M.; Gholami S.; Moazeni M.; Nokhodchi A. Improved Delivery of Voriconazole to Aspergillus Fumigatus through Solid Lipid Nanoparticles as an Effective Carrier. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 558, 338–342. 10.1016/j.colsurfa.2018.08.082. [DOI] [Google Scholar]
- Salehi Z.; Fattahi A.; Lotfali E.; Kazemi A.; Shakeri-Zadeh A.; Nasrollahi S. A. Susceptibility Pattern of Caspofungin-Coated Gold Nanoparticles Against Clinically Important Candida Species. Adv. Pharm. Bull. 2021, 11, 693. 10.34172/apb.2021.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorbakhsh F.; Rezaie S.; Shahverdi A. R.. Antifungal Effects of Silver Nanoparticle Alone and with Combination of Antifungal Drug on Dermatophyte Pathogen Trichophyton Rubrum. In Proceedings of the International conference on bioscience, biochemistry and bioinformatics; 2011; Vol. 5, pp 364–367.
- Lewis D.F. V; Rodrigues A. D.; Ioannides C.; Parke D. V Adverse Reactions of Imidazole Antifungal Agents: Computer Graphic Studies of Cytochrome P-450 Interactions. J. Biochem. Toxicol. 1989, 4, 231–234. 10.1002/jbt.2570040405. [DOI] [PubMed] [Google Scholar]
- Rosenberger A.; Tebbe B.; Treudler R.; Orfanos C. E. Acute Generalized Exanthematous Pustulosis, Induced by Nystatin. Hautarzt. 1998, 49, 492–495. 10.1007/s001050050776. [DOI] [PubMed] [Google Scholar]
- Rai M.; Ingle A. P.; Pandit R.; Paralikar P.; Gupta I.; Anasane N.; Dolenc-Voljč M.. Nanotechnology for the Treatment of Fungal Infections on Human Skin. In The microbiology of skin, soft tissue, bone and joint infections; Elsevier, 2017; pp 169–184. [Google Scholar]
- Wakelin S. H.; Maibach H. I.; Archer C. B.. Handbook of Systemic Drug Treatment in Dermatology; CRC Press, 2023. [Google Scholar]
- Hussain A.; Samad A.; Singh S. K.; Ahsan M. N.; Haque M. W.; Faruk A.; Ahmed F. J. Nanoemulsion Gel-Based Topical Delivery of an Antifungal Drug: In Vitro Activity and in Vivo Evaluation. Drug Delivery 2016, 23, 642–657. 10.3109/10717544.2014.933284. [DOI] [PubMed] [Google Scholar]
- Kassem M. A. A.; Esmat S.; Bendas E. R.; El-Komy M. H. M. Efficacy of Topical Griseofulvin in Treatment of Tinea Corporis. Mycoses 2006, 49, 232–235. 10.1111/j.1439-0507.2006.01221.x. [DOI] [PubMed] [Google Scholar]
- Chen Y.-C.; Liu D.-Z.; Liu J.-J.; Chang T.-W.; Ho H.-O.; Sheu M.-T. Development of Terbinafine Solid Lipid Nanoparticles as a Topical Delivery System. Int. J. Nanomedicine 2012, 7, 4409. 10.2147/IJN.S33682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waghule T.; Sankar S.; Rapalli V. K.; Gorantla S.; Dubey S. K.; Chellappan D. K.; Dua K.; Singhvi G. Emerging Role of Nanocarriers Based Topical Delivery of Anti-fungal Agents in Combating Growing Fungal Infections. Dermatol. Ther. 2020, 33, e13905 10.1111/dth.13905. [DOI] [PubMed] [Google Scholar]
- Kumar M.; Mahmood S.; Mandal U. K. An Updated Account On Formulations And Strategies For The Treatment Of Burn Infection-A Review. Curr. Pharm. Des. 2022, 28, 1480. 10.2174/1381612828666220519145859. [DOI] [PubMed] [Google Scholar]
- Gupta P.; Hafeez A.; Kushwaha P. Development and Evaluation of Topical Ethosomal Gel for Fungal Infections. Drug Res. (Stuttg). 2023, 73, 46–53. 10.1055/a-1924-7818. [DOI] [PubMed] [Google Scholar]
- El-Hadidy G. N.; Ibrahim H. K.; Mohamed M. I.; El-Milligi M. F. Microemulsions as Vehicles for Topical Administration of Voriconazole: Formulation and in Vitro Evaluation. Drug Dev. Ind. Pharm. 2012, 38, 64–72. 10.3109/03639045.2011.590731. [DOI] [PubMed] [Google Scholar]
- Patel M. R.; Patel R. B.; Parikh J. R.; Solanki A. B.; Patel B. G. Investigating Effect of Microemulsion Components: In Vitro Permeation of Ketoconazole. Pharm. Dev. Technol. 2011, 16, 250–258. 10.3109/10837451003610845. [DOI] [PubMed] [Google Scholar]
- Promjan S.; Boonme P. Itraconazole-Loaded Microemulsions: Formulation, Characterization, and Dermal Delivery Using Shed Snakeskin as the Model Membrane. Pharm. Dev. Technol. 2023, 28, 51–60. 10.1080/10837450.2022.2162082. [DOI] [PubMed] [Google Scholar]
- Oerlemans C.; Bult W.; Bos M.; Storm G.; Nijsen J. F. W.; Hennink W. E. Polymeric Micelles in Anticancer Therapy: Targeting, Imaging and Triggered Release. Pharm. Res. 2010, 27, 2569–2589. 10.1007/s11095-010-0233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachhav Y. G.; Mondon K.; Kalia Y. N.; Gurny R.; Möller M. Novel Micelle Formulations to Increase Cutaneous Bioavailability of Azole Antifungals. J. Control. release 2011, 153, 126–132. 10.1016/j.jconrel.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Purohit D. K.Nano-Lipid Carriers for Topical Application: Current Scenario. Asian J. Pharm. 2016, 10. 10.22377/ajp.v10i1.544 [DOI] [Google Scholar]
- Gupta M.; Agrawal U.; Vyas S. P. Nanocarrier-Based Topical Drug Delivery for the Treatment of Skin Diseases. Expert Opin. Drug Delivery 2012, 9, 783–804. 10.1517/17425247.2012.686490. [DOI] [PubMed] [Google Scholar]
- Yang T. L.; Hsieh C. M.; Meng L. J.; Tsai T.; Chen C. T. Oleic Acid-Based Self Micro-Emulsifying Delivery System for Enhancing Antifungal Activities of Clotrimazole. Pharmaceutics 2022, 14, 478. 10.3390/pharmaceutics14030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A.; Sharma U. S. Liposomes in Drug Delivery: Progress and Limitations. Int. J. Pharm. 1997, 154, 123–140. 10.1016/S0378-5173(97)00135-X. [DOI] [Google Scholar]
- Agarwal R.; Katare O. P. Preparation and in Vitro Evaluation of Miconazole Nitrate-Loaded Topical Liposomes. Pharm. Technol. North Am. 2002, 26, 48–60. [Google Scholar]
- Guo F.; Wang J.; Ma M.; Tan F.; Li N. Skin Targeted Lipid Vesicles as Novel Nano-Carrier of Ketoconazole: Characterization, in Vitro and in Vivo Evaluation. J. Mater. Sci. Mater. Med. 2015, 26, 1–13. 10.1007/s10856-015-5487-2. [DOI] [PubMed] [Google Scholar]
- Patel R. P.; Patel H.; Baria A. H. Formulation and Evaluation of Liposomes of Ketoconazole. Int. J. Drug Deliv Technol. 2009, 1, 16–23. 10.25258/ijddt.v1i1.8834. [DOI] [Google Scholar]
- Firthouse P. U. M.; Halith S. M.; Wahab S. U.; Sirajudeen M.; Mohideen S. K. Formulation and Evaluation of Miconazole Niosomes. Int. J. PharmTech Res. 2011, 3, 1019–1022. [Google Scholar]
- Shirsand S. B.; Para M. S.; Nagendrakumar D.; Kanani K. M.; Keerthy D. Formulation and Evaluation of Ketoconazole Niosomal Gel Drug Delivery System. Int. J. Pharm. Investig. 2012, 2, 201. 10.4103/2230-973X.107002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta M.; Vyas S. P. Development, Characterization and in Vivo Assessment of Effective Lipidic Nanoparticles for Dermal Delivery of Fluconazole against Cutaneous Candidiasis. Chem. Phys. Lipids 2012, 165, 454–461. 10.1016/j.chemphyslip.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Maheshwari R. G. S.; Tekade R. K.; Sharma P. A.; Darwhekar G.; Tyagi A.; Patel R. P.; Jain D. K. Ethosomes and Ultradeformable Liposomes for Transdermal Delivery of Clotrimazole: A Comparative Assessment. Saudi Pharm. J. 2012, 20, 161–170. 10.1016/j.jsps.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma P.; Pathak K. Nanosized Ethanolic Vesicles Loaded with Econazole Nitrate for the Treatment of Deep Fungal Infections through Topical Gel Formulation. Nanomedicine Nanotechnology, Biol. Med. 2012, 8, 489–496. 10.1016/j.nano.2011.07.004. [DOI] [PubMed] [Google Scholar]
- El-Sonbaty M. M.; Akl M. A.; El-Say K. M.; Kassem A. A. Does the Technical Methodology Influence the Quality Attributes and the Potential of Skin Permeation of Luliconazole Loaded Transethosomes?. J. Drug Delivery Sci. Technol. 2022, 68, 103096. 10.1016/j.jddst.2022.103096. [DOI] [Google Scholar]
- Bhalaria M. K.; Naik S.; Misra A. N. Ethosomes: A Novel Delivery System for Antifungal Drugs in the Treatment of Topical Fungal Diseases. Indian J. Exp. Biol. 2009, 47 (5), 368–75. [PubMed] [Google Scholar]
- Singh S.; Verma D.; Mirza M. A.; Das A. K.; Anwer M. K.; Sultana Y.; Talegaonkar S.; Iqbal Z. Development and Optimization of Ketoconazole Loaded Nano-Transfersomal Gel for Vaginal Delivery Using Box-Behnken Design: In Vitro, Ex Vivo Characterization and Antimicrobial Evaluation. J. Drug Delivery Sci. Technol. 2017, 39, 95–103. 10.1016/j.jddst.2017.03.007. [DOI] [Google Scholar]
- Ali S. S.; Gudipati M.; Nadendla R. Development and Characterization of Miconazole Nitrate Transfersomal Gel. Int. J. Res. Pharm. Sci. Technol. 2020, 1, 109–116. 10.33974/ijrpst.v1i4.200. [DOI] [Google Scholar]
- Sharma G.; Thakur K.; Raza K.; Singh B.; Katare O. P. Nanostructured Lipid Carriers: A New Paradigm in Topical Delivery for Dermal and Transdermal Applications. Crit. Rev. Ther. Drug Carr. Syst. 2017, 34, 355–386. 10.1615/CritRevTherDrugCarrierSyst.2017019047. [DOI] [PubMed] [Google Scholar]
- Chen Y. C.; Liu D. Z.; Liu J. J.; Chang T. W.; Ho H. O.; Sheu M. T. Development of Terbinafine Solid Lipid Nanoparticles as a Topical Delivery System. Int. J. Nanomedicine. 2012, 7, 4409. 10.2147/IJN.S33682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalekar M. R.; Pokharkar V.; Madgulkar A.; Patil N.; Patil N. Preparation and Evaluation of Miconazole Nitrate-Loaded Solid Lipid Nanoparticles for Topical Delivery. Aaps Pharmscitech 2009, 10, 289–296. 10.1208/s12249-009-9199-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S.; Ray S.; Thakur R. S. Design and evaluation of itraconazole loaded solid lipid nanoparticilate system for improving the antifungal therapy. Pak. J. Pharm. Sci. 2009, 22, 131–138. [PubMed] [Google Scholar]
- Elshaer E. E.; Elwakil B. H.; Eskandrani A.; Elshewemi S. S.; Olama Z. A. Novel Clotrimazole and Vitis Vinifera Loaded Chitosan Nanoparticles: Antifungal and Wound Healing Efficiencies. Saudi J. Biol. Sci. 2022, 29, 1832–1841. 10.1016/j.sjbs.2021.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose A.; Nabi B.; Rehman S.; Md S.; Alhakamy N. A.; Ahmad O. A. A.; Baboota S.; Ali J. Development and Evaluation of Polymeric Nanosponge Hydrogel for Terbinafine Hydrochloride: Statistical Optimization, In Vitro and In Vivo Studies. Polymers (Basel). 2020, 12, 2903. 10.3390/polym12122903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özcan I.; Abacı O.; Uztan A. H.; Aksu B.; Boyacıoglu H.; Güneri T.; Özer O. Enhanced Topical Delivery of Terbinafine Hydrochloride with Chitosan Hydrogels. Aaps Pharmscitech 2009, 10, 1024–1031. 10.1208/s12249-009-9299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparajay P.; Dev A. Development and Evaluation of Eberconazole-Loaded Niosomes. Chem. Proc. 2021, 8, 28. 10.3390/ecsoc-25-11664. [DOI] [Google Scholar]
- Bothiraja C.; Gholap A. D.; Shaikh K. S.; Pawar A. P. Investigation of Ethyl Cellulose Microsponge Gel for Topical Delivery of Eberconazole Nitrate for Fungal Therapy. Ther. Delivery 2014, 5, 781–794. 10.4155/tde.14.43. [DOI] [PubMed] [Google Scholar]
- Sudradjat S. E.; Mun’im A.; Timotius K. H.; Anwar E.. Formulation and Penetration Study of Etosom Gel Myristicin of Nutmeg Oil (Myristica Fragrans H.). The 2nd International Conference on Herbal and Traditional Medicine; Green Journal, 2017.
- Sudhakar B.; Varma J. N.; Murthy K. V. Formulation, Characterization and Ex Vivo Studies of Terbinafine HCl Liposomes for Cutaneous Delivery. Curr. Drug Delivery 2014, 11, 521–530. 10.2174/1567201810666140109113830. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Li X.; Zhu S.; Zhang T.; Maimaiti A.; Ding M.; Shi S. Dermal Targeting Delivery of Terbinafine Hydrochloride Using Novel Multi-Ethosomes: A New Approach to Fungal Infection Treatment. Coatings 2020, 10, 304. 10.3390/coatings10040304. [DOI] [Google Scholar]
- Bhattacharya S. Preparation and Characterizations of Glyceryl Oleate Ufasomes of Terbinafine Hydrochloride: A Novel Approach to Trigger Candida Albicans Fungal Infection. Futur. J. Pharm. Sci. 2021, 7, 1–11. 10.1186/s43094-020-00143-w. [DOI] [Google Scholar]
- Mahoto M.; Tyagi Y.; Ashok P. K. Development and evaluation of nanoemulsion loaded gel of terbinafine hydrochloride for the treatment of fungal infections. W. J. Pharm. Res. 2021, 10, 130–1371. [Google Scholar]
- Basha M.; Abd El-Alim S. H.; Shamma R. N.; Awad G. E. A. Design and Optimization of Surfactant-Based Nanovesicles for Ocular Delivery of Clotrimazole. J. Liposome Res. 2013, 23, 203–210. 10.3109/08982104.2013.788025. [DOI] [PubMed] [Google Scholar]
- Ning M.; Guo Y.; Pan H.; Chen X.; Gu Z. Preparation, in Vitro and in Vivo Evaluation of Liposomal/Niosomal Gel Delivery Systems for Clotrimazole. Drug Dev. Ind. Pharm. 2005, 31, 375–383. 10.1081/DDC-54315. [DOI] [PubMed] [Google Scholar]
- Souto E. B.; Wissing S. A.; Barbosa C. M.; Müller R. H. Development of a Controlled Release Formulation Based on SLN and NLC for Topical Clotrimazole Delivery. Int. J. Pharm. 2004, 278, 71–77. 10.1016/j.ijpharm.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Bolla P. K.; Meraz C. A.; Rodriguez V. A.; Deaguero I.; Singh M.; Yellepeddi V. K.; Renukuntla J. Clotrimazole Loaded Ufosomes for Topical Delivery: Formulation Development and in-Vitro Studies. Molecules 2019, 24, 3139. 10.3390/molecules24173139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marto J.; Vitor C.; Guerreiro A.; Severino C.; Eleutério C.; Ascenso A.; Simões S. Ethosomes for Enhanced Skin Delivery of Griseofulvin. Colloids Surfaces B Biointerfaces 2016, 146, 616–623. 10.1016/j.colsurfb.2016.07.021. [DOI] [PubMed] [Google Scholar]
- Aggarwal N.; Goindi S. Preparation and Evaluation of Antifungal Efficacy of Griseofulvin Loaded Deformable Membrane Vesicles in Optimized Guinea Pig Model of Microsporum Canis—Dermatophytosis. Int. J. Pharm. 2012, 437, 277–287. 10.1016/j.ijpharm.2012.08.015. [DOI] [PubMed] [Google Scholar]
- Bavarsad N.; Kouchak M.; Mohamadipour P.; Sadeghi-Nejad B. Preparation and Physicochemical Characterization of Topical Chitosan-Based Film Containing Griseofulvin-Loaded Liposomes. J. Adv. Pharm. Technol. Res. 2016, 7, 91. 10.4103/2231-4040.184591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal N. R.; Patel H. N.; Mehta L. S.; Sanghani R. M.; Lundberg G. P.; Lewis S. J.; Mendelson M. A.; Wood M. J.; Volgman A. S.; Mieres J. H. Sex Differences in Ischemic Heart Disease: Advances, Obstacles, and next Steps. Circ. Cardiovasc. Qual. Outcomes 2018, 11, e004437 10.1161/CIRCOUTCOMES.117.004437. [DOI] [PubMed] [Google Scholar]
- Jadon P. S.; Gajbhiye V.; Jadon R. S.; Gajbhiye K. R.; Ganesh N. Enhanced Oral Bioavailability of Griseofulvin via Niosomes. Aaps Pharmscitech 2009, 10, 1186–1192. 10.1208/s12249-009-9325-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal N.; Goindi S. Preparation and in Vivo Evaluation of Solid Lipid Nanoparticles of Griseofulvin for Dermal Use. J. Biomed. Nanotechnol. 2013, 9, 564–576. 10.1166/jbn.2013.1569. [DOI] [PubMed] [Google Scholar]
- Barakat H. S.; Darwish I. A.; El-Khordagui L. K.; Khalafallah N. M. Development of Naftifine Hydrochloride Alcohol-Free Niosome Gel. Drug Dev. Ind. Pharm. 2009, 35, 631–637. 10.1080/03639040802498864. [DOI] [PubMed] [Google Scholar]
- ESENTURK GUZEL I. improved skin penetration and deposition of naftifine from transethosomes and transethosomal gel formulations. Farmacia 2022, 70, 514–521. 10.31925/farmacia.2022.3.18. [DOI] [Google Scholar]
- Erdal M. S.; Özhan G.; Mat M. C.; Özsoy Y.; Güngör S. Colloidal Nanocarriers for the Enhanced Cutaneous Delivery of Naftifine: Characterization Studies and in Vitro and in Vivo Evaluations. Int. J. Nanomedicine 2016, 11, 1027. 10.2147/IJN.S96243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.; Rathore P.; Shukla M.; Nayak S. Comparative Studies on Skin Permeation of Miconazole Using Different Novel Carriers. Int. J. Pharm. Sci. Res. 2010, 1, 61–66. 10.13040/IJPSR.0975-8232.1(9-S).61-66. [DOI] [Google Scholar]
- Pandit J.; Garg M.; Jain N. K. Miconazole Nitrate Bearing Ultraflexible Liposomes for the Treatment of Fungal Infection. J. Liposome Res. 2014, 24, 163–169. 10.3109/08982104.2013.871025. [DOI] [PubMed] [Google Scholar]
- Imam S. S.; Gilani S. J.; Zafar A.; Jumah M. N. B.; Alshehri S. Formulation of Miconazole-Loaded Chitosan-Carbopol Vesicular Gel: Optimization to In Vitro Characterization, Irritation, and Antifungal Assessment. Pharmaceutics 2023, 15, 581. 10.3390/pharmaceutics15020581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayah D. Y.; Eid A. M. Development of Miconazole Nitrate Nanoparticles Loaded in Nanoemulgel to Improve Its Antifungal Activity. Saudi Pharm. J. 2023, 31, 526–534. 10.1016/j.jsps.2023.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmoslemany R. M.; Abdallah O. Y.; El-Khordagui L. K.; Khalafallah N. M. Propylene Glycol Liposomes as a Topical Delivery System for Miconazole Nitrate: Comparison with Conventional Liposomes. AAPS PharmSciTech 2012, 13, 723–731. 10.1208/s12249-012-9783-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed S. H.; Tirumoorthy N.; Kundlik G. Enhanced Transdermal Delivery of Ketoconazole via Ethosomes Formulation and Evaluation. World J. Pharm. Pharm. Sci. 2007, 1, 238–249. [Google Scholar]
- Aljohani A. A.; Alanazi M. A.; Munahhi L. A.; Hamroon J. D.; Mortagi Y.; Qushawy M.; Soliman G. M. Binary Ethosomes for the Enhanced Topical Delivery and Antifungal Efficacy of Ketoconazole. OpenNano 2023, 11, 100145. 10.1016/j.onano.2023.100145. [DOI] [Google Scholar]
- Dave V.; Sharma S.; Yadav R. B.; Agarwal U. Herbal Liposome for the Topical Delivery of Ketoconazole for the Effective Treatment of Seborrheic Dermatitis. Appl. Nanosci. 2017, 7, 973–987. 10.1007/s13204-017-0634-3. [DOI] [Google Scholar]
- Lalvand M.; Hashemi S. J.; Bayat M. Effect of Fluconazole and Terbinafine Nanoparticles on the Treatment of Dermatophytosis Induced by Trichophyton Mentagrophytes in Guinea Pig. Iran. J. Microbiol. 2021, 13, 608. 10.18502/ijm.v13i5.7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolge H.; Patil G.; Jadhav S.; Ghormade V. A PH-Tuned Chitosan-PLGA Nanocarrier for Fluconazole Delivery Reduces Toxicity and Improves Efficacy against Resistant Candida. Int. J. Biol. Macromol. 2023, 227, 453–461. 10.1016/j.ijbiomac.2022.12.139. [DOI] [PubMed] [Google Scholar]
- El-Housiny S.; Shams Eldeen M. A.; El-Attar Y. A.; Salem H. A.; Attia D.; Bendas E. R.; El-Nabarawi M. A. Fluconazole-Loaded Solid Lipid Nanoparticles Topical Gel for Treatment of Pityriasis Versicolor: Formulation and Clinical Study. Drug Delivery 2018, 25, 78–90. 10.1080/10717544.2017.1413444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra K. K.; Kaur C. D.; Gupta A. Development of Itraconazole Loaded Ultra-Deformable Transethosomes Containing Oleic-Acid for Effective Treatment of Dermatophytosis: Box-Behnken Design, Ex-Vivo and in-Vivo Studies. J. Drug Delivery Sci. Technol. 2022, 67, 102998. 10.1016/j.jddst.2021.102998. [DOI] [Google Scholar]
- ElMeshad A. N.; Mohsen A. M. Enhanced Corneal Permeation and Antimycotic Activity of Itraconazole against Candida Albicans via a Novel Nanosystem Vesicle. Drug Delivery 2016, 23, 2115–2123. 10.3109/10717544.2014.942811. [DOI] [PubMed] [Google Scholar]
- Mahmoud R. A.; Hussein A. K.; Nasef G. A.; Mansour H. F. Oxiconazole Nitrate Solid Lipid Nanoparticles: Formulation, in-Vitro Characterization and Clinical Assessment of an Analogous Loaded Carbopol Gel. Drug Dev. Ind. Pharm. 2020, 46, 706–716. 10.1080/03639045.2020.1752707. [DOI] [PubMed] [Google Scholar]
- Khan W. A.; Sharma V.; Maurya P.; Bijauliya R. K. Development and Characterization of Oxiconazole Nitrate Loaded Ethosomal Gel for Treating Fungal Infections. World J. Pharmacol Res. 2019, 8, 1341–1356. 10.20959/wjpr201910-15708. [DOI] [Google Scholar]
- Baghel S.; Nair V. S.; Pirani A.; Sravani A. B.; Bhemisetty B.; Ananthamurthy K.; Aranjani J. M.; Lewis S. A. Luliconazole-loaded Nanostructured Lipid Carriers for Topical Treatment of Superficial Tinea Infections. Dermatol. Ther. 2020, 33, e13959 10.1111/dth.13959. [DOI] [PubMed] [Google Scholar]
- Mahmood A.; Rapalli V. K.; Gorantla S.; Waghule T.; Singhvi G. Dermatokinetic Assessment of Luliconazole-Loaded Nanostructured Lipid Carriers (NLCs) for Topical Delivery: QbD-Driven Design, Optimization, and in Vitro and Ex Vivo Evaluations. Drug Delivery Transl. Res. 2022, 12, 1118–1135. 10.1007/s13346-021-00986-7. [DOI] [PubMed] [Google Scholar]
- Alhakamy N. A.; Md S.; Alam M. S.; Shaik R. A.; Ahmad J.; Ahmad A.; Kutbi H. I.; Noor A. O.; Bagalagel A.; Bannan D. F. Development, Optimization, and Evaluation of Luliconazole Nanoemulgel for the Treatment of Fungal Infection. J. Chem. 2021, 2021, 1. 10.1155/2021/4942659. [DOI] [Google Scholar]
- Maibach H. I.; Luo E. C.; Hsu T.-M.. Topical Administration of Basic Antifungal Compositions to Treat Fungal Infections of the Nails. U.S. Patent US6846837B2, 2005.
- Baker J. R. Jr; Flack M. R.; Ciotti S. M.; Sutcliffe J. A.. Methods of Treating Fungal, Yeast and Mold Infections. U.S. Patent US20090269380A1, 2012.
- Blank L. B.; Gettings R. L.; White W. C.. Method of Treating Tinea Pedis and Related Dermatophytic Infections. U.S. Patent US4865844A, 1989.
- Godefroi E. F.; Eijcken C. A. M. V.. Composition and Method for Combating Fungus with Imidazole Carboxylates. U.S. Patent US3485917A, 1969.
- Brillowska-Dabrowska A. H.Pcr Diagnostics of Dermatophytes and Other Pathogenic Fungi. Patent WO2006133701A2, 2010.
- Pier A. C.Broad Spectrum Dermatophyte Vaccine. U.S. Patent US5277904A, 1994.
- Gray N. M.; Woosley R. L.. Methods Ad Compositions of (−) Ketoconazole for Treating Fungal Yeast and Dermatophyte Infections. Patent WO1994014447A1, 2000.
- Freeman A.; Segal R.; Dror Y.. Methods and Compositions for Treating Fungal Infections. U.S. Patent US7825104B2, 2010.
- Scoppettuolo L.; Peterson M.; Almarsson O.; Remenar J.. Novel Saperconazole Crystalline Forms and Related Processes, Pharmaceutical Compositions and Methods. U.S. Patent US20070293674A1, 2007.
- Balkovec J. M.; Bartizal K.; Locke J. B.; Ong V.; Sandison T.; Thye D.; Perlin D. S.; James K. D.. Methods for Treating Fungal Infections. U.S. Patent US20180256673A1, 2018.
- Khanuja S. P. S.; Chaturvedi P.; Singh A. K.; Shasany A. K.; Agarwal V. K.; Gupta V. K.; Gupta S. C.; Tripathy A. K.; Pal A.; Saikia D.. Anti-Dermatophytic Preparation and Use Thereof. U.S. Patent US7291349B2, 2007.
- Trimble J. O.Antifungal Treatment of Nails. U.S. Patent US8333981B2, 2012.
- Genberg C.; Beus C. S.; Savage P. B.. Methods for Treating Fungal Infections. U.S. Patent US10238665B2, 2019.
- Bartizal K.; Daruwala P.. Methods for Treating Fungal Infections. U.S. Patent US20190374601A1, 2019.
- Zhou B.Treatment of Fungal Infection by Light Irradiation. U.S. Patent US20120310307A1, 2012.
- Usman M.; Farooq M.; Wakeel A.; Nawaz A.; Cheema S. A.; ur Rehman H.; Ashraf I.; Sanaullah M. Nanotechnology in Agriculture: Current Status, Challenges and Future Opportunities. Sci. Total Environ. 2020, 721, 137778. 10.1016/j.scitotenv.2020.137778. [DOI] [PubMed] [Google Scholar]
- Kumar M.; Dogra R.; Mandal U. K. Nanomaterial-Based Delivery of Vaccine through Nasal Route: Opportunities, Challenges, Advantages, and Limitations. J. Drug Delivery Sci. Technol. 2022, 74, 103533. 10.1016/j.jddst.2022.103533. [DOI] [Google Scholar]
- Murray A. R.; Kisin E. R.; Tkach A. V.; Yanamala N.; Mercer R.; Young S.-H.; Fadeel B.; Kagan V. E.; Shvedova A. A. Factoring-in Agglomeration of Carbon Nanotubes and Nanofibers for Better Prediction of Their Toxicity versus Asbestos. Part. Fibre Toxicol. 2012, 9, 1–19. 10.1186/1743-8977-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidgeon N.; Harthorn B.; Satterfield T. Nanotechnology Risk Perceptions and Communication: Emerging Technologies, Emerging Challenges. Risk Anal. An Int. J. 2011, 31, 1694–1700. 10.1111/j.1539-6924.2011.01738.x. [DOI] [PubMed] [Google Scholar]
- Romig A. D. Nanotechnology: Scientific Challenges and Societal Benefits and Risks. Metall. Mater. Trans. A 2004, 35, 3641–3648. 10.1007/s11661-004-0270-x. [DOI] [Google Scholar]
- Harshitha C.; Bhattacharyya S. A BRIEF REVIEW ON NANOTECHNOLOGY AS A CHALLENGING FIELD IN PHARMACEUTICALS AND THEIR REGULATORY APPROVAL. J. Crit. Rev. 2020, 7, 963–968. 10.31838/jcr.07.09.178. [DOI] [Google Scholar]
- Tripathy N.; Hong T.-K.; Ha K.-T.; Jeong H.-S.; Hahn Y.-B. Effect of ZnO Nanoparticles Aggregation on the Toxicity in RAW 264.7 Murine Macrophage. J. Hazard. Mater. 2014, 270, 110–117. 10.1016/j.jhazmat.2014.01.043. [DOI] [PubMed] [Google Scholar]
- Moriello K. A.; Coyner K.. Dermatophytosis. In Greene’s Infectious Diseases of the Dog and Cat; Elsevier, 2021; pp 961–977. [Google Scholar]
- Begum J.; Mir N. A.; Lingaraju M. C.; Buyamayum B.; Dev K. Recent Advances in the Diagnosis of Dermatophytosis. J. Basic Microbiol. 2020, 60, 293–303. 10.1002/jobm.201900675. [DOI] [PubMed] [Google Scholar]
- Pakshir K.; Bahaedinie L.; Rezaei Z.; Sodaifi M.; Zomorodian K. In Vitro Activity of Six Antifungal Drugs against Clinically Important Dermatophytes. Jundishapur J. Microbiol. 2008, 2, 158–163. 10.1016/j.ijid.2008.05.762. [DOI] [Google Scholar]
- Gupta A. K.; Tu L. Q. Dermatophytes: Diagnosis and Treatment. J. Am. Acad. Dermatol. 2006, 54, 1050–1055. 10.1016/j.jaad.2006.01.016. [DOI] [PubMed] [Google Scholar]
- AL-Khikani F. H. O.; Ayit A. S. Major Challenges in Dermatophytosis Treatment: Current Options and Future Visions. Egypt. J. Dermatology Venerol. 2021, 41, 1. 10.4103/ejdv.ejdv_23_20. [DOI] [Google Scholar]
- Hänel H.; Braun B.; Jovic N.. Comparative Activity of a Liposomal and a Conventional Econazole Preparation for Topical Use According to a Guinea Pig Tinea Model. In Proceedings of the Liposome Dermatics: Griesbach Conference; Springer, 1992; pp 251–257. [Google Scholar]