Abstract
Intercellular communication is a key feature of cancer progression and metastasis. Extracellular vesicles (EVs) are generated by all cells, including cancer cells and recent studies have identified EVs as key mediators of cell-cell communication via packaging and transfer of bioactive constituents to impact the biology and function of cancer cells and cells of the tumor microenvironment. Here, we review recent advances in understanding the functional contribution of EVs to cancer progression and metastasis, as cancer biomarkers, and the development of cancer therapeutics.
Introduction
Cancer initiation and progression is facilitated by communication between emerging pre-neoplastic/malignant cells and other cells within the tumor, along with host cells within the local tissue and the entire body. Intercellular communication can facilitate microenvironment changes to influence tumor growth and dissemination of cancer cells. Such signaling can occur through secretion of soluble factors or exchange of extracellular vesicles (EVs). EV secretion was initially described in reticulocytes and was postulated to be a mechanism for removal of excess membrane proteins 1,2. Additional studies revealed that EVs contain bioactive cargo including proteins, lipids, metabolites, RNA, and DNA that can potentially be transferred to recipient cells to impact their function providing evidence that EVs may act as mediators of intercellular communication. Bidirectional communication mediated by EVs has been identified between numerous cell types within the primary and metastatic tumor microenvironment. EVs have pleotropic roles in processes critical for cancer progression, potentially reflective of their heterogeneous origins and constituents. In addition, the accumulation of EVs in tumors, EV biocompatibility and the ability to readily modify EV cargo have been exploited to develop novel EV based therapeutics that target multiple aspects of the tumor microenvironment for therapeutic benefit. In this review, we summarize current knowledge of the function of EVs in cancer initiation, progression, metastasis, and response to therapy, as biomarkers, and in the development cancer therapeutics.
The biology and biogenesis of EVs
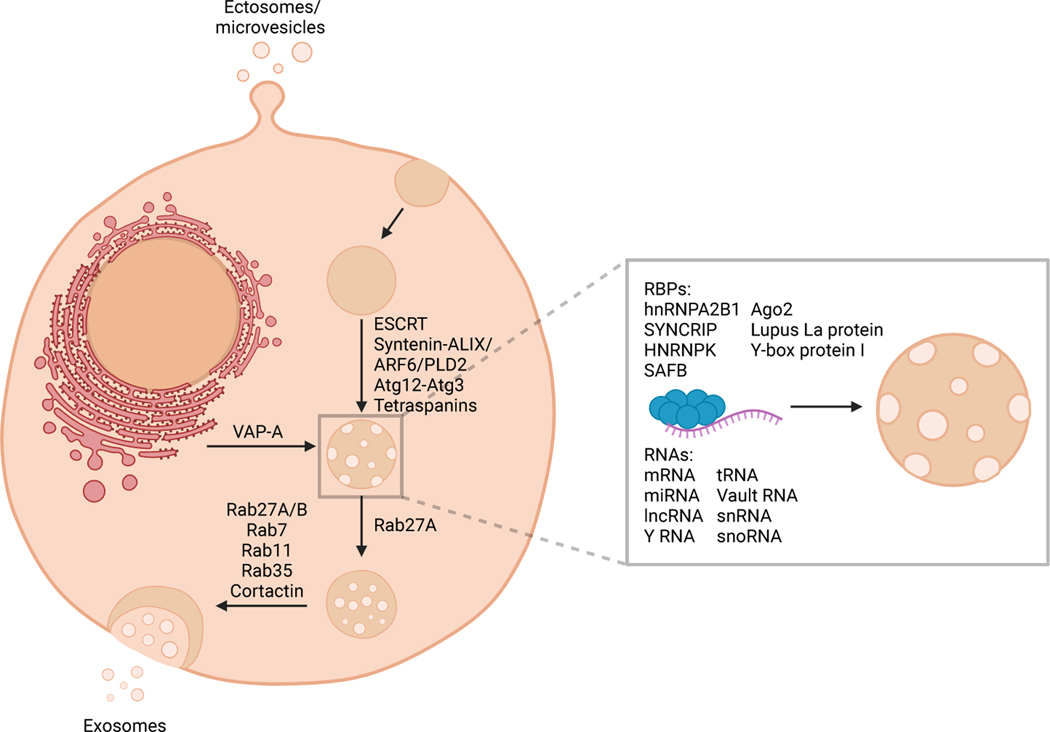
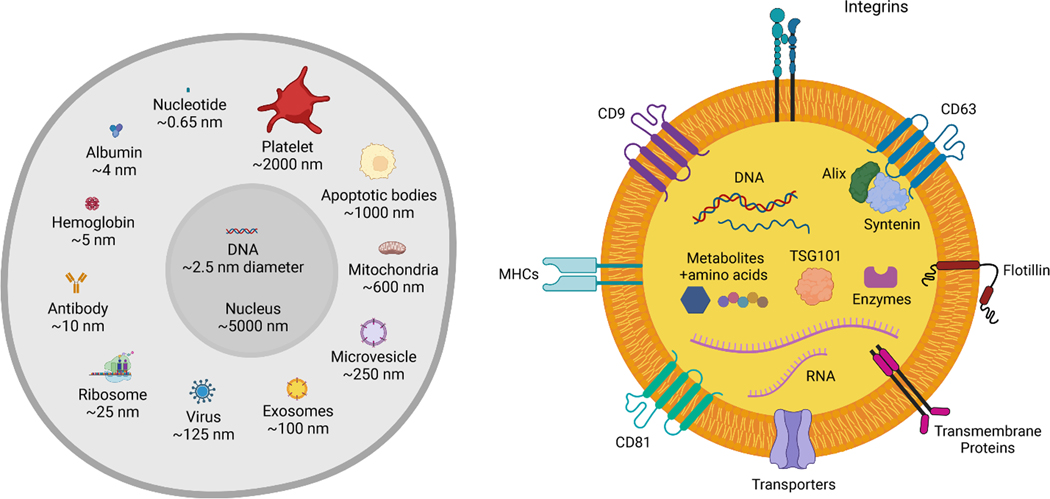
Extracellular vesicles (EVs) consist of two major subsets: exosomes and ectosomes or microvesicles (Figure 1). Exosomes are generally in the size range of 40–150 nm and ectosomes can be in the size range of 50–1000 nm (Figure 2). The tetraspanins CD9, CD63, and CD81, syntenin, integrins, Alix, TSG101, and flotillin are enriched in EVs, although heterogeneity in expression of some EV biomarkers across cell types is observed 3 and overlap in the size and protein expression between ectosomes and exosomes exists. Ectosomes arise from budding at the plasma membrane and are thought to be enriched for CD9 and CD81 4,5 and exosomes are enriched in CD63, CD9, CD81, Alix and syntenin. Isolation of pure populations of ectosomes or exosomes has proved challenging due to overlapping protein marker expression and size. Exosomes are derived from the endocytic pathway, wherein budding of late endosomes leads to formation of intraluminal vesicles within multivesicular bodies (MVBs) that contain biomolecules, including protein, RNA, DNA, lipids, and metabolites 6. The content of EVs is thought to be largely reflective of the cell of origin. Specifically, the metabolic state of the cell can impact EV protein cargo 7, circadian rhythms regulate the packaging of protein into EVs 8, and EVs contain cell type-specific cargo 3. During MVB formation, several biomolecules are incorporated including RNA and protein. Recent models of exosome release suggest that endoplasmic reticulum (ER)-late endosome membrane contact sites regulate late endosome motility, maturation, and association with small GTPases, ultimately impacting the fusion of MVBs with the plasma membrane and release of exosomes 9. Specific RNAs are enriched in EVs compared to their cell of origin, suggesting that selective RNA packaging mechanisms control the RNAs loaded into EVs (Figure 1).
Figure 1. Extracellular vesicles include exosomes and ectosomes or microvesicles.
EVs present with a phospholipid bilayer membrane oriented similarly to that of the plasma membrane of the cell they are generated from. Exosomes are generated via the endosomal pathway, and result via the sequential invagination of the plasma membrane forming multivesicular bodies before they are released extracellularly. Ectosomes/microvesicles are generated via the outward budding of the plasma membrane. The mediators of different stages of multivesicular body formation, maturation, and release and RNA packaging into EVs are labeled. RBPs, RNA binding proteins. Created with BioRender.com.
Figure 2. Relative size and cargo of EVs.
(A) Average size of exosomes and microvesicles with respect to cellular components, including abundant proteins (albumin, hemoglobin, antibody), organelles (ribosomes, mitochondria), nucleotides and DNA, virus, as well as cellular byproducts: apoptotic bodies and platelets. (B) Composite cargo of exosomes, including surface receptors (protein, glycoprotein, glycans, ion channel receptors, G-protein coupled receptors, enzyme-linked receptors, integrins, etc.), transmembrane proteins (FasL, PD-L1, etc.), intracellular proteins, metabolites, lipids, and nucleic acids (RNA: mRNA, pre-/miRNA, piRNA, tRNA, snRNA, snoRNA, Y-RNA, circRNA; DNA: dsDNA, ssDNA, mtDNA, foreign DNA; cAMP). Created with BioRender.com.
The endosomal protein sorting complex (ESCRT) recognizes ubiquitinated cargo and mediates its packaging into MVBs 10. ESCRT components also control MVB size and the protein cargo of secreted exosomes 11, albeit MVB and exosome biogenesis can occur independent of ESCRTs 12. Exosomes are enriched with tetraspanins, including CD9, CD63, and CD81. CD63 13 as well as tetraspanin-enriched microdomains promote the packaging of proteins into exosomes 14. Despite the identification of several mediators of RNA and protein packaging into EVs, the impact of transferred biomolecules on recipient cell behavior is still unclear. Current approaches rely on silencing miRNAs or genes in the EV producing cell, which may have unintended off-target effects on recipient cell behavior. Alternative strategies that target specific RNA and/or protein packaging molecules or incorporate strategies to inhibit components of EVs without impacting other aspects of signaling in the EV producing cell could further clarify this point.
In addition to RNA and proteins, ssDNA, mtDNA, and dsDNA molecules are present in EVs. DNA packaging into EVs was proposed as a mechanism to remove inflammatory cytoplasmic DNA from cells 15,16; however, conflicting reports exist on whether DNA is associated with exosomes or small EVs despite employing similar EV isolation techniques 17,18, suggesting that DNA packaging in EVs may be cell type dependent or that DNA is in low abundance in EVs, limiting its detection. Nonetheless, other studies propose that nuclear content including dsDNA can be packaged into EVs through micronuclei 19. Alternatively, FLAP/5-lipoxygenase+ EVs can arise at the nuclear envelope through nSMase1-dependent ceramide synthesis 20.
MVBs that fuse to the plasma membrane release exosomes into the extracellular space, which can then be transferred to recipient cells and potentially impact their function. Rab GTPases regulate vesicle budding and motility to facilitate the trafficking of MVBs for exosome release (Figure 1) 21. The endosomal pathway is also linked to autophagy as MVBs can fuse with autophagosomes for lysosomal degradation, indicating that autophagy mediators also function in exosome secretion. At the plasma membrane, cortactin in conjunction with Rab27a facilitates MVB docking and exosome release 22 and the composition of the glycocalyx can drive plasma membrane instabilities to facilitate EV secretion 23.
Other nonvesicular nanoparticles that are secreted by cells have also been identified, including exomeres and supermeres. While the precise mechanisms regulating the secretion of exomeres and supermeres remain unknown and whether they are just an aggregated collection of proteins need to be clarified. Exomeres and supermeres appear to be different from small EVs or exosomes based on their size (~45 nm and ~35 nm, respectively). Exomeres are reported to have distinct proteomic profiles and biodistribution patterns compared to small EVs 24, whereas supermeres are enriched with RNAs and have increased accumulation in tissues compared to exomeres and small EVs 25. Exomeres from MDCK cells are associated with amphiregulin (AREG), which regulates EGFR trafficking in intestinal organoids 26. Supermeres from colorectal cancer cells are reported to impact lactate secretion and can transfer cetuximab resistance to non-resistant cells 25. Additional insight into the cargo and physiological functions of extracellular particles is likely to be gained as the biogenesis and biology of such particles is unraveled. Moreover, the majority of studies to understand EV function employ ex vivo isolated EVs and/or bolus administration of EVs. As a result, the physiological role of EV exchange in vivo remains largely unknown and new models that enable fate mapping and tracking endogenous EV release (discussed in more detail in the perspectives and future directions section) and the discovery of more specific mediators of EV secretion will further clarify EV function.
The function of EVs in development
Intercellular communication across cells and tissues is required for proper tissue patterning and development, and many developmental processes are activated in the context of cancer to promote progression. Blastocysts secrete dsDNA-containing EVs prior to implantation providing a potential non-invasive strategy for monitoring embryos 27, but the functional relevance of DNA in blastocyst EVs and in EVs produced by other cell types remains unknown. EV release is considered to be important for maintaining ESC pluripotency via FAK activation 28, which may be a conserved mechanism of stemness maintenance in embryonic stem cells and adult stem cells, including cancer stem-like cells. Incubation of sperm with EVs derived from stressed epididymal epithelial cells led to offspring with changes in expression of genes related to neurodevelopment and alterations in response to chronic stress 29, suggesting that EVs can transmit information across generations. EV associated dsDNA has 5’-cytosine methylation 30 and proteins identified in histone modification have been identified in EVs 3, raising the possibility that EVs can alter the epigenetic landscape of recipient cells to rewire recipient cell transcription in a more permanent manner. Epigenetic modifications of tumor microenvironment (TME) cells are postulated to play an important role in rewiring TME cell function to promote cancer progression and therapy resistance 31,32. While it is appreciated that EVs are exchanged in the context of development and can act as morphogens 33–35, the regulatory mechanisms that prevent widespread, non-discriminant EV exchange and allow for specific patterning of organs remain to be unraveled. One possibility is that mechanisms limiting the entry of EVs into cells exist, as demonstrated in the context of lung metastasis 36. There is also evidence that internalized EVs can be re-released into the extracellular space 37, which may limit the functional impact of EVs. Alternatively, turnover of delivered EV cargo through degradative mechanisms could lead to transient effects on recipient cells. A better understanding of the fate of EVs and their cargo after internalization will clarify their role in eliciting transient vs. long-term effects in the context of normal physiology and cancer.
EV mediated control of aging and metabolism
Cancer is considered to be a disease of aging, as cancer incidence is higher in older individuals in part due to age-dependent accumulation of somatic mutations, but also a result of mutation-independent mechanisms such as increased inflammation and remodeling of the microenvironment 38. A number of EV-based strategies have been developed to reverse aging phenotypes in vivo. Neonatal umbilical cord mesenchymal stem cell (MSC) derived EVs transfer proliferating cell nuclear antigen (PCNA) to adult bone marrow MSCs and inhibited bone and kidney degeneration associated with aging 39. EVs from young fibroblasts contain GSTM2 which is transferred to aging tissue to increase GSH levels and reduce ROS and lipid peroxidation 40. Thus, EVs may have promise as anti-aging agents that could be repurposed for cancer applications, but a better appreciation of the mediators of EV function in aging and overlapping functions in cancer will provide optimal ways to leverage EVs therapeutically.
Communication between organs shapes the overall metabolic state of organisms. Analysis of EVs from distinct cellular sources revealed tissue-specific proteins, providing potential biomarkers of altered tissue metabolism 41. miRNAs in adipose tissue derived EVs are transferred to the brain to induce damage to synapses and cognitive impairment 42. In adipose tissue, EVs are exchanged between adipocytes and endothelial cells, enabling the transfer of proteins from endothelial cells to adipocytes. Such transfer is regulated by the systemic nutrient state, with fasting increasing endothelial cell EV secretion 43 and exercise increasing the proteome of EVs in circulation 44. While these studies have unraveled the role of EVs in the context of altered metabolic states, the function of EVs in establishing and maintaining metabolic homeostasis remains elusive. Endogenous EV transfer between the brain and pancreas has been reported 42, suggesting that EVs may function in hormone regulation in the context of normal physiology. Moreover, EVs have intrinsic metabolic activity 45, suggesting that they have the capacity to remodel local metabolite abundance. Such EV mediated control of organismal metabolism could have important undiscovered implications in the context of cancer, specifically to mediate metastasis, impact therapeutic responses, and reshape the microbiome.
The impact of EVs on tissue repair, response to stress, and immunity
In the context of damaged tissues and tumors, cells are exposed to many types of stress, including genetic defects, nutrient scarcity, hypoxia, and mechanical stress. Cellular responses to such stresses are pleotropic and context dependent and the same is likely true for EVs. Indeed, EVs have been implicated in facilitating tissue repair and response to stress 46–48. EVs can have both tissue regenerative and destructive properties, and a comprehensive understanding of their function in response to tissue damage and in mediating tissue repair may provide ways to exploit and/or target EV transfer therapeutically.
Cell-cell signaling is critical for eliciting effective immune responses while preventing overexuberant immune activation that can lead to chronic inflammation and autoimmunity which are risk factors for cancer development. Dendritic cell EVs have MHC class II on their surface and can transfer MHC class II/antigen complexes to antigen presenting cells which in turn elicit T cell activation 49–54. Plasmacytoid dendritic cells (pDCs) transfer antigens through EVs to conventional dendritic cells, enabling cross priming of CD8+ T cells 55. EVs, as opposed to donor cells, are the major facilitators of MHC cross-dressing that promotes alloimmune responses to heart and islet transplantation 56. Moreover, knockout of the EV secretion mediators Rab27a and Rab27b leads to chronic inflammation and inhibited responses to inflammatory signals 57, indicating that EVs may play a role in maintaining immunological homeostasis. Targeting of EV secretion by cancer cells has been proposed as a therapeutic target; however, broad targeting of EV secretion of all cells may have unwanted off-target effects that are tumor promoting. Consequently, a broader understanding of the functional contribution of EVs by non-cancer cells will provide critical insight into the feasibility of targeting EV secretion.
EVs serve a critical function in responding to infections and mediating cross-kingdom communication between the host organism and infectious agents. Transmissibility of a number of infectious agents, including HIV, noroviruses, rotaviruses, enteroviruses, malaria, prions, and anthrax, is impacted by EVs 58–63. In the context of infection, IL-35 on Treg derived EVs promotes infectious tolerance by stimulating non-Tregs to produce IL-35 and by promoting B and T cell exhaustion 64. Interactions between the tissue microbiome and immune cells mediated by EVs has been implicated in several inflammatory disorders 65–68. The bidirectional cross talk mediated by EVs between host cells and the microbiome is likely important for tissue homeostasis and in mediating the immune response to inflammatory conditions, including cancer.
The role of EVs in inflammation, obesity, and cancer initiation
Chronic inflammatory disorders such as diabetes, pancreatitis, fibrosis, and non-alcoholic steatophepatitis are all risk factors for cancer development. Pancreatic islet cells release autoantigens in EVs in response to ER stress that stimulate T cell activation 69. β cells secrete miRNAs in EVs in response to cytokines that can induce apoptosis in recipient cells 70. In addition, the proinflammatory β cell EV cargo can lead to dysfunction of recipient β cells and recruitment of macrophages and T cells, promoting disease progression 71. Islet EVs increase the expression of cytokines secreted by Th1, Th2, and Th17 cells and increase the production of autoantibodies associated with type I diabetes 72. Chronic inflammation and fibrosis can modulate the tissue microenvironment to promote cancer initiation. Pancreatitis lead to increased EVs in circulation and such EVs activated macrophages into a pro-inflammatory phenotype 73. Moreover, plasma EVs from patients with severe pancreatitis elicited activation of NFκB signaling, expression of TNFα and IL1β, and generation of free radicals in macrophages 74. Helicobacter pylori, the causative agent of gastritis, produce EVs that stimulate the secretion of TNFα, IL6, and IL1β by macrophages and IL8 by gastric epithelial cells to induce inflammation known to drive tumorigenesis 75.
Obesity is a risk factor for cancer, potentially through inflammation induction 76. In early onset obesity, macrophage derived EVs containing miR-690 and hepatocyte derived EVs containing miR-3075 act to promote insulin sensitivity 77,78. In contrast, in chronic obesity, EVs promote insulin resistance through proinflammatory signaling 77, suggesting that EV release is initially protective and is subjugated in chronic obesity to promote disease progression. High fat diet and high caloric intake drives the initiation of nonalcoholic steatohepatitis (NASH) in mice, which is characterized by excessive fat accumulation, fibrosis, and inflammation in the liver and is a risk factor for developing liver cancer 79. In healthy livers, miR-690 is transferred from Kupffer cells to hepatocytes and stellate cells through EVs and acts to prevent the development of NASH and NASH is associated with loss of miR-690 in Kupffer cells 80. NASH typically leads to lipotoxicity and ER stress that is mediated by inositol-requiring enzyme-1A (IRE1A) 81. IRE1A stimulates the transcription of serine palmitoyltransferase genes to increase the release of hepatocyte derived EVs and drive inflammation 82. Hepatocytes treated with the toxic lipid mediator lysophosphatidylcholine secrete EVs enriched with β1 integrin that increase proinflammatory monocyte adhesion to liver sinusoidal cells 83. Thus, EVs have context-dependent roles in cancer initiation, with both restraining and promoting cancer initiation.
The early genetic drivers of cancer initiation can impact EV secretion, cargo packaging, and entry into recipient cells. Malignant cells typically have higher EV secretion compared to non-malignant cells, which is likely mediated by the mobilization of calcium from the ER 84. Moreover, activation of p53 in response to stress is associated with increased EV secretion through TSAP6 85. The oncogenes AURKB, MYC, and HRASG12V alter EV release, size, and their protein and miRNA composition 86. Mutant RAS also induces the entry of EVs into cancer cells through macropinocytosis 87–89. Cellular transformation with oncogenic HRAS induces the release of EVs containing oncogenic DNA 90; however, the precise impact of EV associated DNA on recipient cells is not fully understood and whether such transfer occurs in vivo is not known. Mutant KRAS inhibits the accumulation of Ago2 in multivesicular endosomes and EVs, modifying the packaging of miRNA in EVs 91. A number of oncogenic miRNA have been identified that have critical roles in tumor initiation and progression 92. Breast cancer EVs are capable of processing precursor miRNAs into mature miRNAs, and transfer of EV associated miRNAs is sufficient to drive the transformation of nontumorigenic epithelial cells 93. While such studies have implicated EVs in processes that may increase the risk of developing cancer, currently evaluating the direct contribution of EVs to tumor initiation is difficult due to a lack of cell lines derived from precursor lesions and no specific EV markers of tumor initiating cells. Models that enable the study of such early lesions will provide clarify this point and provide potential early biomarkers of disease.
The functional contribution of EVs to cancer progression
A signaling network involving cancer cells and non-malignant cells, including epithelial cells, cancer associated fibroblasts (CAFs), endothelial cells, neurons, and immune cells, is critical for driving as well as restraining cancer progression. Transfer of EVs between cancer cells and stromal cells has been identified as a mechanism to reprogram the host tissue to alter tissue homeostasis and aid cancer progression (Supplementary Table 1–2). Pancreatic cancer cell EVs are enriched for biomolecules that elicit ER stress in non-tumorigenic recipient cells, potentially promoting their transformation 94. PTEN is packaged in EVs and transferred between cells to inhibit Akt signaling and proliferation 95, suggesting a mechanism by which EVs from nonmalignant cells limit cancer cell proliferation. A dynamic transfer of EVs between cancer cells and other cells in the TME likely exists, and the balance of cancer cell EV secretion compared to TME cell EV secretion, as well as the cargo of such EVs, could ultimately determine cancer progression. Cancer cells generally have increased EV release compared to non-tumorigenic cells in the context of in vitro two-dimensional tissue culture plastic; however, this remains to be validated in vivo where the tissue microenvironment is more complex.
Cancer cell EVs can also transfer a number of immunomodulatory factors that impact antitumor immunity. Natural killer (NK) cell EVs carry cytotoxic proteins that elicit cancer cell killing which may act to limit cancer progression 96. Multiple myeloma (MM) cells secrete EVs with the NKG2D ligands that initially activate NK cells; however, with prolonged exposure to MM EVs, NKG2D is downregulated leading to hindered NK function 97. This suggests that the initial response to cancer cell EVs may be to induce the antitumor activity of immune cells, but such response can be ultimately subjugated by cancer cells to promote immune escape and disease progression. Cancer cell EVs have been implicated in promoting an immunosuppressive TME through suppression of T cells 98,99 and dendritic cells (DCs) 100,101, and promoting the pro-tumorigenic functions of macrophages 102 and myeloid derived suppressor cells (MDSCs) 103. In contrast, subcapsular sinus CD169+ macrophages internalize cancer cell EVs, preventing their interaction with tumor promoting B cells 104. This suggests that in some instances, EV entry may act as a functional sink preventing the delivery of EV cargo to other cell types. The fate of EV cargo in recipient cells and mechanisms controlling the targeting of EV cargo for degradation as opposed to retention are currently not completely understood.
The communication axis between cancer cells and CAFs mediated by EVs also impacts tumor growth and the immune microenvironment. Cancer cell EVs containing factors such as TGFβ, miR-125b, and mutant gain-of-function p53 are transferred to fibroblasts to induce CAF activation and promote cancer growth 105–108. Activated NOTCH-MYC signaling in CAFs elicits secretion of unshielded RN7SL1 RNA in EVs that is transferred to breast cancer cells, driving expression of the RNA pattern recognition receptor RIG-I and promoting tumor progression 109. It is possible that other stromal cell types can contribute immunogenic RNA associated with EVs and that stromal ssDNA and dsDNA in EVs can elicit innate immune responses; however, this remains to be validated. miR-21, miR-378e, and miR-143 in CAF EVs promote the expression of EMT and cancer stem-like cell genes in breast cancer cells 110. CD9 on CAF EVs is critical for entry into pancreatic cancer cells and pancreatic cancer progression 111; however, pancreatic CAF EVs have also been reported to contain tumor suppressive miRNAs 112, potentially reflecting the functionally heterogeneous populations of CAFs that exist 113. Currently, the contribution of EVs derived from distinct subsets of TME cells remains largely unknown. Experimental models that enable tracking and functionally interrogation of EVs secreted by TME cells will unravel their contribution to cancer progression.
EVs in mediating cancer metastasis
During metastatic progression, cancer cells disseminate from the primary tumor and colonize distant organs. Acquisition of phenotypes that promote escape from the primary tumor, extravasation at secondary sites, and subjugation of the metastatic stroma enable metastasis. In cancer cells that are local invading, EV secretion is increased at invadopodia and such secretion promotes adhesion assembly and is required for directional migration 114,115. Both local and systemic exchange of mRNAs associated with cancer cell EVs occurs, potentially leading to transfer of metastatic behavior between cancer cells 116. Further, the entry of EVs into recipient cells and their impact on cell proliferation is dependent on cancer cell metastatic state 117. Live imaging of zebrafish embryos revealed that cancer cell EVs that are released into circulation enter endothelial cells and macrophages and cancer cell EVs can activate macrophages to facilitate metastatic outgrowth 118. Intravital imaging of EV release and entry in recipient cells in larger scale mammals such as rodents has remained elusive and as a result, the fate of endogenously released EVs in cancer is largely unknown. Advances in imaging technologies may provide methodologies to track EV fate and unravel their functional impact.
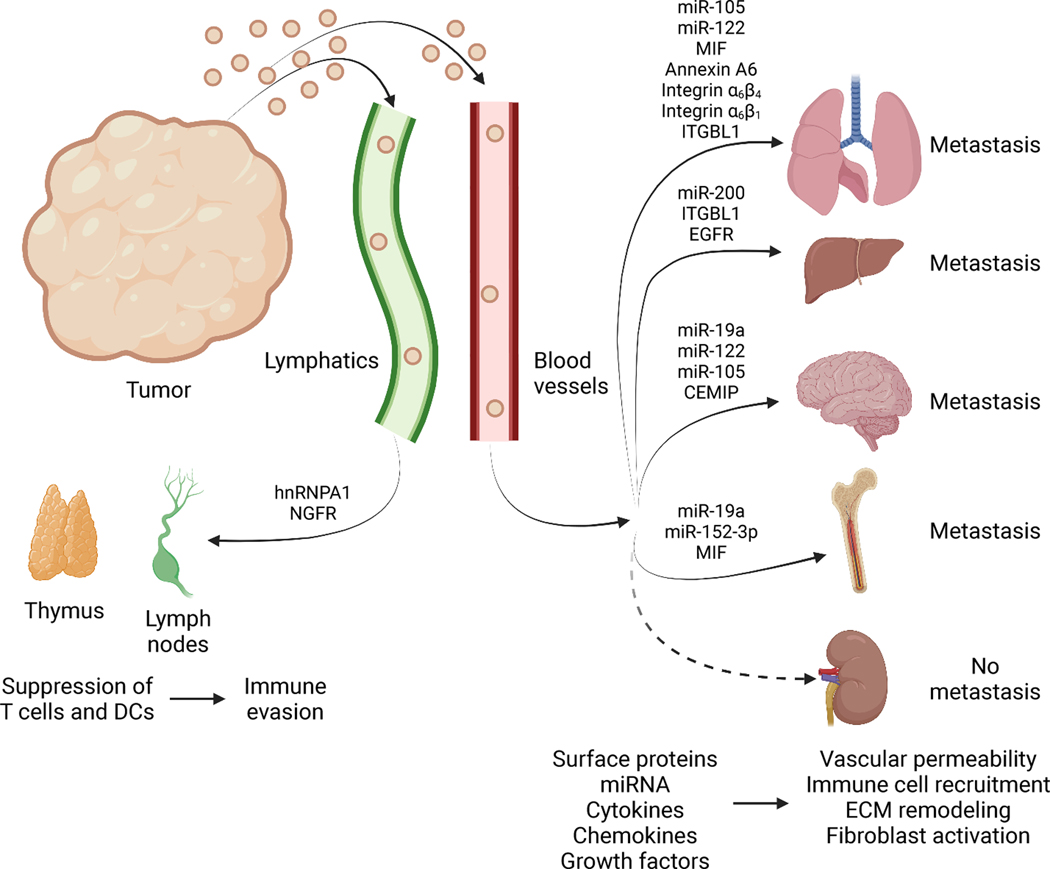
At future metastatic sites, EVs can remodel the microenvironment to create a niche that is permissive for metastatic outgrowth (Figure 3, Supplemental Table 1–2). EVs can impact the vasculature and accumulation of bone marrow derived cells (BMDCs) to enhance metastatic colonization 119,120. In addition to hematogenous spread, cancer cells also initiate metastasis through lymphatics. Melanoma EVs promote ECM deposition and angiogenesis to facilitate metastatic colonization of sentinel lymph nodes 121. There is evidence of tissue-specific accumulation patterns of EVs that are reflective of future sites of metastasis, with EVs expressing α6β4 and α6β1 integrins associated with lung metastasis and αvβ5 integrin associated with liver metastasis 122. While EVs accumulate in sites of metastasis, there is also EV accumulation in organs where metastasis typically does not occur, such as the pancreas, kidney, heart, bladder, and muscle 123. In addition, mechanisms to limit the entry of EVs at metastatic sites have been identified 36,124 and bidirectional communication between cancer cells and the microenvironment is likely critical for metastatic progression. While the role of EVs in promoting metastatic dissemination is well-documented, mechanisms that limit the systemic transfer of EVs are not completely understood and additional metastasis-independent functions of EVs in organs where metastasis does not occur are likely to be uncovered.
Figure 3. Extracellular vesicles in metastatic disease.
Tumors release EVs, from both cancer cells and host cells of the tumor microenvironment (TME) into systemic circulation using both lymphatic and blood vessels. EVs interact with lymphoid organs including thymus and lymph nodes, with impact on T cell activation, DCs, and possibly aiding immune evasion. EVs also influence metastasis to lungs, liver, brain, and bone and possibly other non-metastatic sites by modifying vascular permeability and impacting immune cell recruitment, extracellular matrix (ECM) remodeling, and fibroblast activation. EVs exert their function by altering recipient cells via delivery of RNA, cytokines, chemokines, or growth factors, or surface protein signaling. Created with BioRender.com.
EVs as biomarkers of cancer and therapeutic response
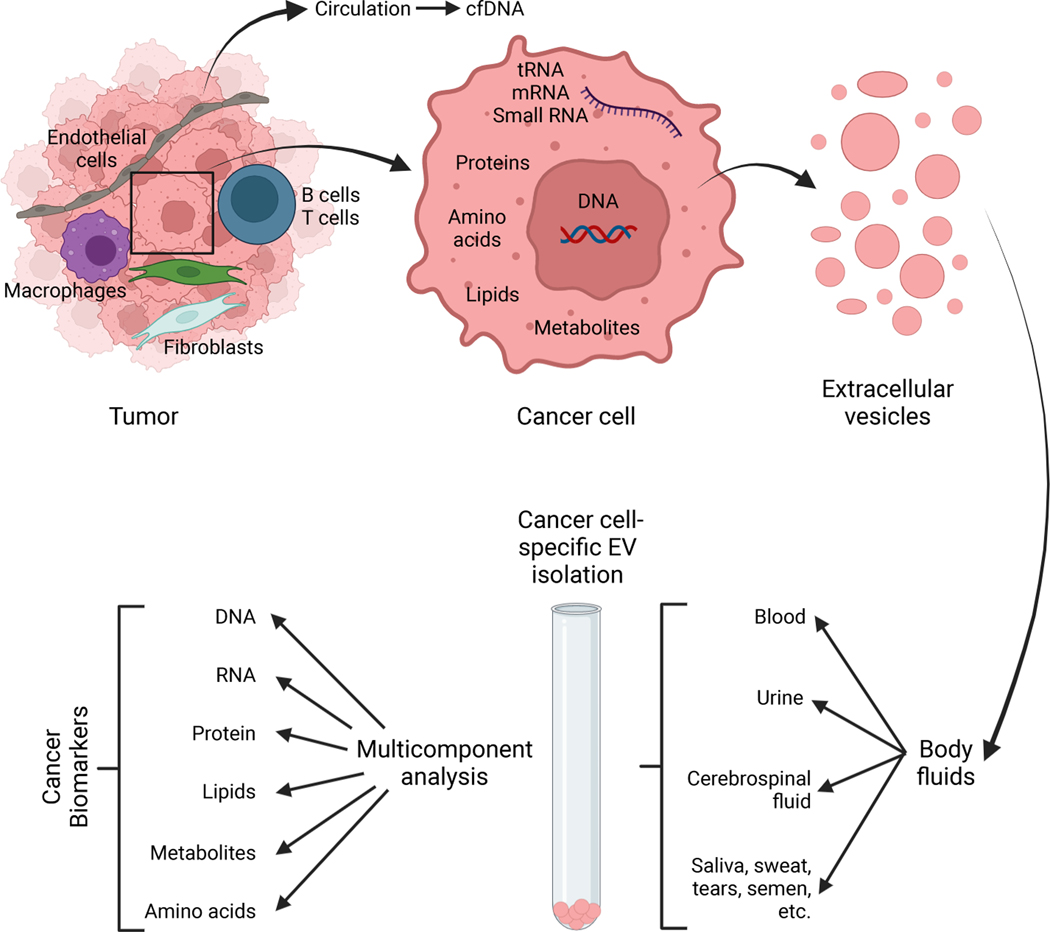
EVs contain nucleic acids, proteins, metabolites, and lipids from the cell of origin and are present in circulation and other bodily fluids, and as a result have emerged as non-invasive biomarkers for disease and response to therapy (Figure 4). A 3 gene expression assay with urine EVs (ExoDx Prostate IntelliScore) can discriminate higher grade prostate cancers (Gleason score 7 or greater) from lower grade tumors and benign disease 125,126 and received a Breakthrough Device Designation by the FDA. Analysis of urine-derived EVs from prostate cancer patients revealed an enrichment of lncRNAs that are predicted to encode high-affinity neoantigens, which may be transferred to recipient cells and translated 127. Moreover, metabolic differences were detected in urine-derived prostate cancer EVs compared to benign prostate hyperplasia EVs, indicating that urinary EVs can be used to non-invasively monitor the metabolic state of prostate tumors 128. Urine EVs have emerged as a source of biomarkers for urological cancers; however, their utility for detection of other cancer types is less known. In addition, EVs derived from stool contain both human and bacterial ribosomal RNA 129, suggesting that stool EVs could be used to non-invasively monitor the evolution of gastrointestinal cancers and the microbiome simultaneously. Future studies evaluating stool EVs as a source of cancer and microbiome biomarkers are needed to determine their feasibility and accuracy for cancer detection and monitoring.
Figure 4. EVs as cancer biomarkers.
Cancer cells shed EVs with a characteristic cargo representing a range of cancer cell components, including nucleic acids, proteins, lipids, metabolites etc. EVs are found in all body fluids, including blood, urine, cerebrospinal fluids, saliva, sweat, tears, semen etc..) and may be enriched with various isolation protocols. EVs lend themselves to a multicomponent analysis reflecting a collection of cancer cells byproducts for biomarkers study, which likely offer a more comprehensive readout when compared to ctDNA analysis alone. Created with BioRender.com.
In addition to RNAs, DNA has been identified in EVs and its utility as a cancer biomarker explored. Common pancreatic cancer cell mutations, including KRASG12D and TRP53R273H, are detected in the DNA derived from EVs in circulation of pancreatic cancer cell patients 30,130–132. Further, glypican 1 (GPC1) was identified as an early-stage marker of pancreatic cancer, and KRAS mutations are detected in GPC1+ EVs 133. While DNA within the lumen of cancer cell EVs has low abundance 18, sequencing of such samples revealed higher coverage, indicating that DNA incorporated in EVs has improved utility compared to other cfDNA isolates.
Proteins present in EVs can enable cancer-specific EV capture and detection. Proteomic analysis of EVs derived from tissue explants, plasma, and bodily fluids identified CD9, HSPA8, ALIX, HSP90AB1, ACTB, MSN, and RAP1B as potential pan-EV markers and VCAN, TNC, and THBS2 as cancer-specific EV markers 134. An advantage of EVs over other biomarkers used for cancer detection and monitoring, including soluble proteins, is that EVs contain multiple biomolecules that can be measured, potentially providing increased sensitivity and specificity. Sensors that simultaneously measure EV proteins and miRNAs as well as protein expression and activity have been developed, allowing for multiplexed analysis of EVs and potentially more accurate detection of cancer EVs 135,136. Improved detection systems that are capable of multiparametric analysis, especially of individual EVs, that are capable of measuring EV heterogeneity are likely to emerge in the future.
EVs have also been evaluated for their utility in tracking responses to therapy. PD-L1 packaged in EVs inhibits T cell activation to promote immunosuppression 98,99 and analysis of plasma EVs from melanoma patients revealed that increases in EV-PD-L1 are associated with disease progression and better predictive value compared to tumor biopsies 137; however, the predictive power of EV based assays is currently limited by the availability of accurate biomarkers. The transcriptional profile of plasma EVs correlates with tumors in melanoma patients and can be used to predict response to immune checkpoint blockade. Deconvolution models were employed to predict the contribution of EVs derived from various melanoma tumor microenvironment cell sources to EVs in circulation 138. Such analyses could be further expanded to profile EVs secreted by cells in the tumor microenvironment and understand their role and predictive power in cancer progression and response to therapy.
Therapeutic responses mediated by EVs
Cancer cells develop a variety of resistance mechanisms in response to therapy, including cell intrinsic and extrinsic mechanisms, and the transfer of miRNAs and lncRNA through EVs can confer chemoresistance to other cancer cells (Supplemental Table 3–4). EV-mediated therapy resistance can potentially act through distinct but not mutually exclusive mechanisms, including transfer of proteins and miRNA that promote therapy resistance 139–141, transfer of drug transporters 142, acting as decoys for antibody-based therapeutics 99, and by preventing antibodies from accessing their ligand target 143. EV secretion is also postulated to be a mechanism of removal of unwanted cellular materials, suggesting that drugs may be packaged into EVs, limiting their functional impact on cancer cells. The lncRNA lncARSR (lncRNA Activated in RCC with Sunitinib Resistance) is incorporated in sunitinib resistant RCC EVs and can transmit resistance by competitively binding miR-34/miR-449 to induce AXL and c-MET expression 144. EVs from GBM cells transfer spliceosomal proteins and snRNA to recipient cells to impact transcription in recipient cells, promoting therapy resistance 145. Cargo packaged in EVs from stromal cells, including CAFs, endothelial cells, and immune cells, have been implicated in therapy resistance. Noncoding RNA and transposable elements in CAF EVs are transferred to breast cancer cells, where they induce pattern recognition and antiviral signaling and activate NOTCH3 to promote therapy resistance 146. Moreover, CAF EVs contain mitochondrial DNA that is transferred to cancer cells to induce oxidative phosphorylation, an escape from dormancy, and resistance to hormone therapy in breast cancer 147.The relative contribution of EVs and their cargo acting as decoys to influence therapeutic responses in comparison to direct transfer of EV cargo to therapy resistance is not currently known and warrants future investigation.
An effective therapy response elicits lasting antitumor immunity and crosstalk between cell compartments in the tumor microenvironment is critical for establishing such memory responses. Vitamin E treatment enhanced DC function through inhibition of SHP1 and increased antigen presentation by DCs and DC derived EVs to elicit antitumor immunity 148, suggesting that EV release can be modulated to promote effective therapy responses. While CAR-T cells have demonstrated effective control of a number of hematopoietic cancers, solid tumors are largely non-responsive to CAR-T cell therapy, in part due to microenvironment factors 149. CAR-T cells engineered to express RN7SL1 transfer EVs containing RN7SL1 RNA to myeloid cells to inhibit the MDSC phenotype and to DCs to promote costimulation, promoting CD8 T cell mediated clearance of solid tumors 150. This suggests that the efficacy of adoptive cell transfer (ACT) therapies is in part dependent on EV transfer, creating a potential opportunity to improve ACT through modulation of EVs.
The development and clinical testing of EV-based cancer therapeutics
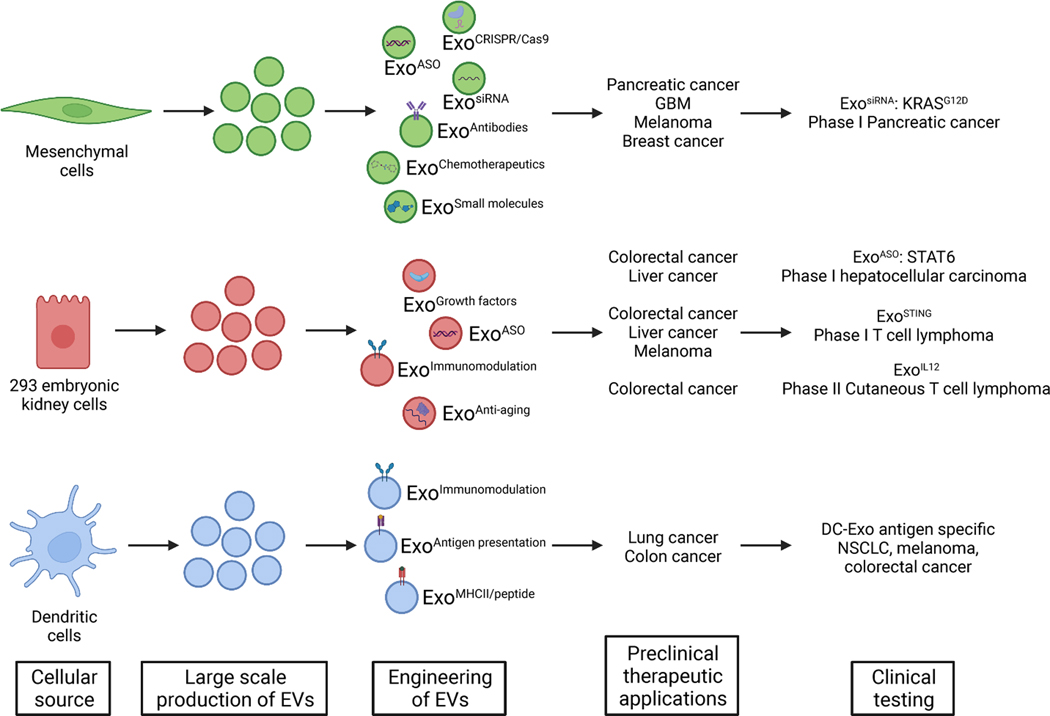
The membrane of EVs can protect intraluminal cargo and the surface proteins in unmodified EVs act to prolong circulation times and accumulation in specific organs, especially tumors and the liver and spleen 88,151. Moreover, EVs are large enough to presumably avoid renal clearance 152. As a result, a number of small molecule drugs have been incorporated into EVs for delivery of therapeutic payload to tumors while limiting off-target effects (Figure 5). The chemotherapeutics paclitaxel, doxorubicin, and gemcitabine have been packaged in EVs and demonstrated effective suppression of tumor growth 153–158. In addition, EV based delivery vehicles with siRNAs targeting KRASG12D, MYC, S100A4, and PAK4 have been employed 88,151,159–161. EVs engineered to incorporate antisense oligonucleotides 162, CRISPR/Cas9 163–165, and miRNA 166,167 have also elicited effective tumor growth control. In order to further improve loading of cargo into EVs while maintaining the biocompatible properties of EVs, hybrid vesicles incorporating both EVs and synthetic materials have been developed 155,168–172. In depth analysis of the immune responses and off-target effects of such strategies will provide critical insight into the clinical feasibility of hybrid and other nanovesicles. In addition, while these engineering strategies may improve cargo loading or targeting to specific tissues, therapeutics with increasing complexity can also create additional regulatory hurdles for clinical translation. As a result, the tradeoffs between such hurdles and engineering benefits need to be weighed for the successful implementation of EV based therapeutics.
Figure 5. EVs as anti-cancer therapeutic agents.
Distinct cellular sources have been used to generate EVs in large scale for clinical trials. EVs engineering include the incorporation of a cargo (e.g. ASO, siRNA, chemotherapeutics etc), enriching for exosomes with unique surface protein presentation (e.g. antigen, immune modifying receptor). Preclinical studies in various tumor models and tumor types informed ongoing clinical trial design. EVs offer a novel therapeutic platform for cancer treatment, from personalized medicine to immunotherapy and targeted therapy with novel safety and efficacy profiles. Created with BioRender.com.
EVs can modulate immune cell function and consequently exploiting such interactions therapeutically has been proposed for the control of cancer progression. STING agonists have demonstrated the ability to stimulate anti-tumor immune responses; however, clinical translation of STING agonists have been limited by bioavailability issues and off-target toxicity. Incorporation of small molecule STING agonists in EVs effectively activates antigen presenting cells and anti-tumor immunity with lack of off-target effects 173,174. Stimulation of the RIG-I pathway leads to type I interferon secretion and an anti-tumor response, but RIG-I agonists have similar limitations to STING agonists in vivo. Incorporation of RIG-I agonists in red blood cell EVs stimulates immune responses and suppresses tumor growth 175, further demonstrating the potential for incorporating immune modulatory molecules in EVs.
A number of cell-based immunotherapies have been developed and demonstrate effective control of tumor growth, including DC vaccines and CAR T cells. Despite their efficacy, cell-based therapies have several limitations, such as development of immunosuppressive mechanisms, off-target toxicities, and the need for autologous cells. EVs have emerged as cell-free immunotherapies that can circumvent many of the issues associated with cell-based therapies. DC derived EVs contain functional MHC class I/peptide complexes which can prime T cells to elicit anti-tumor responses 176. As a result, DC EVs have been proposed as cell-free vaccines for cancer. Small EVs or exosomes from ovalbumin-pulsed dendritic cells induce antigen-specific CD8+ T cells, whereas large EVs or microvesicles do not 177, indicating that small EVs are more effective at eliciting antigen-specific immune responses. EVs derived from DCs pulsed with a cancer-specific aberrant transcription induced chimeric RNA, potentially providing an EV based vaccination strategy for cancers that lack a known mutational antigen 178. Such strategies could be expanded to readily modified EVs to incorporate RNA molecules and/or proteins to vaccinate against various cancer mutations. In addition to DC EVs based vaccination strategies, EVs from other immune cell types have been investigated as cancer therapeutics. EVs from CAR-T cells (CAR-EVs) express CAR on their surface and are capable of inducing cytotoxicity and tumor growth inhibition 179, suggesting that CAR-EVs can act as cell-free immunotherapies. Although CAR-EVs have therapeutic promise, whether autologous EVs are required to prevent graft-versus-host responses is not currently known. Allogenic NK CAR cell therapies have been employed to circumvent this issue and EVs from NK cells contain cytotoxic proteins and demonstrate cancer cell killing capacity 96, suggesting that EVs from NK CAR cells may be an effective immunotherapy. Together, these studies provide strategies for controlling tumor progression via off-the-shelf EV based immunotherapies.
The translation of EV based therapeutic vehicles requires large scale, GMP production. Bioreactors enable large scale culture of cells under defined conditions for EV isolation. Several strategies have been employed to generate clinical-grade EVs based on differential ultracentrifugation 151, density gradient ultracentrifugation 180, and tangential flow filtration (TFF) 181. TFF and size exclusion chromatography (SEC) allow for isolation of EVs from larger volumes of cell culture media, potentially more readily enabling broad clinical application of EV based therapeutics. EV based therapeutics face many of the same challenges associated with the clinical translation of cell-based therapeutics, including characterization of the cellular source of EVs, EV isolation and storage, and quality control and standardization. Phase I trials of DC EV cancer vaccines (Dex) concluded with lack of toxicity and an objective response in one patient 182. In non-small cell lung cancer, a phase II clinical trial of Dex concluded that Dex is well tolerated but did not meet its primary endpoint of 50% of patients with progression-free survival at 4 months post-chemotherapy 183. More recently, several EV based therapeutics for cancer initiated clinical testing. Two phase I trials evaluating EVs incorporating STING agonists (exoSTING, NCT04592484) and IL-12 (exoIL-12, NCT05156229) completed recently. exoIL-12 demonstrated a manageable safety profile in healthy subjects and cutaneous T cell lymphoma patients and the recommended phase 2 dose was identified (NCT05156229). The safety and tolerability of EVs with STAT6 ASOs (exoASO-STAT6) are currently being evaluated in patients with hepatocellular carcinoma and gastric and colorectal cancer metastasis to the liver (NCT05375604). In addition, the safety and efficacy of EVs with KRASG12D targeting siRNA (iExosomes) are being determined in ongoing phase I trials in metastatic pancreatic cancer patients (NCT03608631). Thus far, EV cancer therapeutics appear to be safe and well tolerated, and ongoing trials will provide additional insight into the efficacy of different EV therapeutic modalities.
Perspectives and future directions
Significant advances have been made in recent years that have enabled unprecedented insight into EV biology and function in cancer progression, response to therapy, and metastasis. Our understanding of the function of EVs is predominantly in perturbed systems, i.e. disease states, and the role of EVs in normal physiology and homeostatic tissue function remains elusive. Moreover, precancerous cell types are typically difficult to expand ex vivo and maintain their phenotypes, precluding EV isolation and analysis to evaluate EV contribution to cancer initiation. Similar challenges exist with certain cells in the tumor microenvironment, e.g., lymphatic endothelial cells, neurons, and subsets of immune cells and CAFs. Markers enriched in circulating EVs from early-stage cancer patients and normal individuals have been identified; however, the precise cellular origin of such EVs are not known. Consequently, models that enable the tracking of EVs released by distinct cell populations in vivo will help to clarify these points. In addition, several mediators of EV biogenesis have been identified in vitro, but whether these functions are conserved in vivo and restricted to EV secretion are unknown. The identification of EV-restricted mediators of secretion will more readily enable the functional dissection of the contribution of EVs to cancer progression.
Single EV analysis techniques revealed that individual EVs display heterogeneity in their size 184 and marker expression 185–187; however, the majority of studies focused on understanding the role in EVs in cancer are based on EVs isolated using crude methods that presumably capture a mixture of heterogenous EVs. EVs have differential impacts on metastatic outgrowth and biodistribution based on bulk measured surface markers and size 24,122 and CD63+ EVs contain both common and non-overlapping protein cargo compared to CD9+ EVs 5. Consequently, distinct functional subsets of EVs likely exist. EV technologies have expanded rapidly in recent years to include single EV analysis and sorting as well as methods to isolate EVs based on their size and charge, which will enable the profiling of EV subsets and evaluating their functional role. Moreover, the development of novel techniques to measure nucleic acids in single EVs will further elucidate EV heterogeneity.
Clinical trials of EV based therapeutics thus far have not revealed significant toxicities and unmodified EVs from certain nonmalignant cell types are immunologically inert and can be used as allogenic therapeutics 151,188. Modification of EV cargo to express CD3 antibodies reprograms EVs to activate T cells 189, suggesting that EVs could be further engineered to generate off-the-shelf allogenic therapeutics with defined immune-targeting and/or immunomodulatory properties. as vaccines 190 a strategy which could be further exploited to generate EV vaccines with cancer antigens. Such strategy could be used for personalized medicine to target patient-specific mutations as well as more broadly occurring mutations such as KRASG12D.
Supplementary Material
Acknowledgments
The EV work in the Kalluri lab is supported by MD Anderson Cancer Center, NIH R35CA263815, and NIH P40OD024628 and gifts from Fifth Generation (Love, Tito’s), Lyda Hill Philanthropies®, and Bosarge Family Trust.
Footnotes
Declaration of interests
MD Anderson Cancer Center and R.K. hold patents in the area of exosome biology and are licensed to Codiak Biosciences, Inc. MD Anderson Cancer Center and R.K. are stock equity holders in Codiak Biosciences, Inc. R.K. is a consultant and scientific adviser for Codiak Biosciences, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johnstone RM, Adam M, Hammond JR, Orr L, and Turbide C. (1987). Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 262, 9412–9420. [PubMed] [Google Scholar]
- 2.Pan BT, Teng K, Wu C, Adam M, and Johnstone RM (1985). Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol 101, 942–948. 10.1083/jcb.101.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kugeratski FG, Hodge K, Lilla S, McAndrews KM, Zhou X, Hwang RF, Zanivan S, and Kalluri R. (2021). Quantitative proteomics identifies the core proteome of exosomes with syntenin-1 as the highest abundant protein and a putative universal biomarker. Nat Cell Biol 23, 631–641. 10.1038/s41556-021-00693-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, and Thery C. (2016). Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A 113, E968–977. 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathieu M, Nevo N, Jouve M, Valenzuela JI, Maurin M, Verweij FJ, Palmulli R, Lankar D, Dingli F, Loew D, et al. (2021). Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat Commun 12, 4389. 10.1038/s41467-021-24384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalluri R, and LeBleu VS (2020). The biology, function, and biomedical applications of exosomes. Science 367. 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harada Y, Nakajima K, Suzuki T, Fukushige T, Kondo K, Seino J, Ohkawa Y, Suzuki T, Inoue H, Kanekura T, et al. (2020). Glycometabolic Regulation of the Biogenesis of Small Extracellular Vesicles. Cell Rep 33, 108261. 10.1016/j.celrep.2020.108261. [DOI] [PubMed] [Google Scholar]
- 8.Yeung CC, Dondelinger F, Schoof EM, Georg B, Lu Y, Zheng Z, Zhang J, Hannibal J, Fahrenkrug J, and Kjaer M. (2022). Circadian regulation of protein cargo in extracellular vesicles. Sci Adv 8, eabc9061. 10.1126/sciadv.abc9061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verweij FJ, Bebelman MP, George AE, Couty M, Becot A, Palmulli R, Heiligenstein X, Sires-Campos J, Raposo G, Pegtel DM, and van Niel G. (2022). ER membrane contact sites support endosomal small GTPase conversion for exosome secretion. J Cell Biol 221. 10.1083/jcb.202112032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katzmann DJ, Babst M, and Emr SD (2001). Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell 106, 145–155. 10.1016/s0092-8674(01)00434-2. [DOI] [PubMed] [Google Scholar]
- 11.Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, Manel N, Moita LF, Thery C, and Raposo G. (2013). Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci 126, 5553–5565. 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- 12.Stuffers S, Sem Wegner C, Stenmark H, and Brech A. (2009). Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic 10, 925–937. 10.1111/j.1600-0854.2009.00920.x. [DOI] [PubMed] [Google Scholar]
- 13.van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, Marks MS, Rubinstein E, and Raposo G. (2011). The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell 21, 708–721. 10.1016/j.devcel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perez-Hernandez D, Gutierrez-Vazquez C, Jorge I, Lopez-Martin S, Ursa A, Sanchez-Madrid F, Vazquez J, and Yanez-Mo M. (2013). The intracellular interactome of tetraspanin-enriched microdomains reveals their function as sorting machineries toward exosomes. J Biol Chem 288, 11649–11661. 10.1074/jbc.M112.445304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi A, Okada R, Nagao K, Kawamata Y, Hanyu A, Yoshimoto S, Takasugi M, Watanabe S, Kanemaki MT, Obuse C, and Hara E. (2017). Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat Commun 8, 15287. 10.1038/ncomms15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clancy JW, Sheehan CS, Boomgarden AC, and D’Souza-Schorey C. (2022). Recruitment of DNA to tumor-derived microvesicles. Cell Rep 38, 110443. 10.1016/j.celrep.2022.110443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, Liebler DC, Ping J, Liu Q, Evans R, et al. (2019). Reassessment of Exosome Composition. Cell 177, 428–445 e418. 10.1016/j.cell.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lazaro-Ibanez E, Lasser C, Shelke GV, Crescitelli R, Jang SC, Cvjetkovic A, Garcia-Rodriguez A, and Lotvall J. (2019). DNA analysis of low- and high-density fractions defines heterogeneous subpopulations of small extracellular vesicles based on their DNA cargo and topology. J Extracell Vesicles 8, 1656993. 10.1080/20013078.2019.1656993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yokoi A, Villar-Prados A, Oliphint PA, Zhang J, Song X, De Hoff P, Morey R, Liu J, Roszik J, Clise-Dwyer K, et al. (2019). Mechanisms of nuclear content loading to exosomes. Sci Adv 5, eaax8849. 10.1126/sciadv.aax8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arya SB, Chen S, Jordan-Javed F, and Parent CA (2022). Ceramide-rich microdomains facilitate nuclear envelope budding for non-conventional exosome formation. Nat Cell Biol 24, 1019–1028. 10.1038/s41556-022-00934-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stenmark H. (2009). Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 10, 513–525. 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 22.Sinha S, Hoshino D, Hong NH, Kirkbride KC, Grega-Larson NE, Seiki M, Tyska MJ, and Weaver AM (2016). Cortactin promotes exosome secretion by controlling branched actin dynamics. J Cell Biol 214, 197–213. 10.1083/jcb.201601025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shurer CR, Kuo JC, Roberts LM, Gandhi JG, Colville MJ, Enoki TA, Pan H, Su J, Noble JM, Hollander MJ, et al. (2019). Physical Principles of Membrane Shape Regulation by the Glycocalyx. Cell 177, 1757–1770 e1721. 10.1016/j.cell.2019.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang H, Freitas D, Kim HS, Fabijanic K., Li Z, Chen H, Mark MT, Molina H, Martin AB, Bojmar L, et al. (2018). Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol 20, 332–343. 10.1038/s41556-018-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Q, Jeppesen DK, Higginbotham JN, Graves-Deal R, Trinh VQ, Ramirez MA, Sohn Y, Neininger AC, Taneja N, McKinley ET, et al. (2021). Supermeres are functional extracellular nanoparticles replete with disease biomarkers and therapeutic targets. Nat Cell Biol 23, 1240–1254. 10.1038/s41556-021-00805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Q, Higginbotham JN, Jeppesen DK, Yang YP, Li W, McKinley ET, Graves-Deal R, Ping J, Britain CM, Dorsett KA, et al. (2019). Transfer of Functional Cargo in Exomeres. Cell Rep 27, 940–954 e946. 10.1016/j.celrep.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simon B, Bolumar D, Amadoz A, Jimenez-Almazan J, Valbuena D, Vilella F, and Moreno I. (2020). Identification and Characterization of Extracellular Vesicles and Its DNA Cargo Secreted During Murine Embryo Development. Genes (Basel) 11. 10.3390/genes11020203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hur YH, Feng S, Wilson KF, Cerione RA, and Antonyak MA (2021). Embryonic Stem Cell-Derived Extracellular Vesicles Maintain ESC Stemness by Activating FAK. Dev Cell 56, 277–291 e276. 10.1016/j.devcel.2020.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan JC, Morgan CP, Adrian Leu N, Shetty A, Cisse YM, Nugent BM, Morrison KE, Jasarevic E, Huang W, Kanyuch N, et al. (2020). Reproductive tract extracellular vesicles are sufficient to transmit intergenerational stress and program neurodevelopment. Nat Commun 11, 1499. 10.1038/s41467-020-15305-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thakur BK, Zhang H, Becker A, Matei I, Huang Y, Costa-Silva B, Zheng Y, Hoshino A, Brazier H, Xiang J, et al. (2014). Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res 24, 766–769. 10.1038/cr.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee YT, Tan YJ, Falasca M, and Oon CE (2020). Cancer-Associated Fibroblasts: Epigenetic Regulation and Therapeutic Intervention in Breast Cancer. Cancers (Basel) 12. 10.3390/cancers12102949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henning AN, Roychoudhuri R, and Restifo NP (2018). Epigenetic control of CD8(+) T cell differentiation. Nat Rev Immunol 18, 340–356. 10.1038/nri.2017.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gross JC, Chaudhary V, Bartscherer K, and Boutros M. (2012). Active Wnt proteins are secreted on exosomes. Nat Cell Biol 14, 1036–1045. 10.1038/ncb2574. [DOI] [PubMed] [Google Scholar]
- 34.Korkut C, Ataman B, Ramachandran P, Ashley J, Barria R, Gherbesi N, and Budnik V. (2009). Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell 139, 393–404. 10.1016/j.cell.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gradilla AC, Gonzalez E, Seijo I, Andres G, Bischoff M, Gonzalez-Mendez L, Sanchez V, Callejo A, Ibanez C, Guerra M, et al. (2014). Exosomes as Hedgehog carriers in cytoneme-mediated transport and secretion. Nat Commun 5, 5649. 10.1038/ncomms6649. [DOI] [PubMed] [Google Scholar]
- 36.Ortiz A, Gui J, Zahedi F, Yu P, Cho C, Bhattacharya S, Carbone CJ, Yu Q, Katlinski KV, Katlinskaya YV, et al. (2019). An Interferon-Driven Oxysterol-Based Defense against Tumor-Derived Extracellular Vesicles. Cancer Cell 35, 33–45 e36. 10.1016/j.ccell.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Brien K, Ughetto S, Mahjoum S, Nair AV, and Breakefield XO (2022). Uptake, functionality, and re-release of extracellular vesicle-encapsulated cargo. Cell Rep 39, 110651. 10.1016/j.celrep.2022.110651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collado M, Blasco MA, and Serrano M. (2007). Cellular senescence in cancer and aging. Cell 130, 223–233. 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Lei Q, Gao F, Liu T, Ren W, Chen L, Cao Y, Chen W, Guo S, Zhang Q, Chen W, et al. (2021). Extracellular vesicles deposit PCNA to rejuvenate aged bone marrow-derived mesenchymal stem cells and slow age-related degeneration. Sci Transl Med 13. 10.1126/scitranslmed.aaz8697. [DOI] [PubMed] [Google Scholar]
- 40.Fafian-Labora JA, Rodriguez-Navarro JA, and O’Loghlen A. (2020). Small Extracellular Vesicles Have GST Activity and Ameliorate Senescence-Related Tissue Damage. Cell Metab 32, 71–86 e75. 10.1016/j.cmet.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia-Martin R, Brandao BB, Thomou T, Altindis E, and Kahn CR (2022). Tissue differences in the exosomal/small extracellular vesicle proteome and their potential as indicators of altered tissue metabolism. Cell Rep 38, 110277. 10.1016/j.celrep.2021.110277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J, Li L, Zhang Z, Zhang X, Zhu Y, Zhang C, and Bi Y. (2022). Extracellular vesicles mediate the communication of adipose tissue with brain and promote cognitive impairment associated with insulin resistance. Cell Metab 34, 1264–1279 e1268. 10.1016/j.cmet.2022.08.004. [DOI] [PubMed] [Google Scholar]
- 43.Crewe C, Joffin N, Rutkowski JM, Kim M, Zhang F, Towler DA, Gordillo R, and Scherer PE (2018). An Endothelial-to-Adipocyte Extracellular Vesicle Axis Governed by Metabolic State. Cell 175, 695–708 e613. 10.1016/j.cell.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whitham M, Parker BL, Friedrichsen M, Hingst JR, Hjorth M, Hughes WE, Egan CL, Cron L, Watt KI, Kuchel RP, et al. (2018). Extracellular Vesicles Provide a Means for Tissue Crosstalk during Exercise. Cell Metab 27, 237–251 e234. 10.1016/j.cmet.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 45.Iraci N, Gaude E, Leonardi T, Costa ASH, Cossetti C, Peruzzotti-Jametti L, Bernstock JD, Saini HK, Gelati M, Vescovi AL, et al. (2017). Extracellular vesicles are independent metabolic units with asparaginase activity. Nat Chem Biol 13, 951–955. 10.1038/nchembio.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fry CS, Kirby TJ, Kosmac K, McCarthy JJ, and Peterson CA (2017). Myogenic Progenitor Cells Control Extracellular Matrix Production by Fibroblasts during Skeletal Muscle Hypertrophy. Cell Stem Cell 20, 56–69. 10.1016/j.stem.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kfoury YS, Ji F, Mazzola M, Sykes DB, Scherer AK, Anselmo A, Akiyama Y, Mercier F, Severe N, Kokkaliaris KD, et al. (2021). tiRNA signaling via stress-regulated vesicle transfer in the hematopoietic niche. Cell Stem Cell 28, 2090–2103 e2099. 10.1016/j.stem.2021.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu J, Wu X, Lu J, Huang G, Dang L, Zhang H, Zhong C, Zhang Z, Li D, Li F, et al. (2021). Exosomal transfer of osteoclast-derived miRNAs to chondrocytes contributes to osteoarthritis progression. Nature Aging 1, 368–384. 10.1038/s43587-021-00050-6. [DOI] [PubMed] [Google Scholar]
- 49.Utsugi-Kobukai S, Fujimaki H, Hotta C, Nakazawa M, and Minami M. (2003). MHC class I-mediated exogenous antigen presentation by exosomes secreted from immature and mature bone marrow derived dendritic cells. Immunol Lett 89, 125–131. 10.1016/s0165-2478(03)00128-7. [DOI] [PubMed] [Google Scholar]
- 50.Thery C, Duban L, Segura E, Veron P, Lantz O, and Amigorena S. (2002). Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol 3, 1156–1162. 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 51.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, and Geuze HJ (1996). B lymphocytes secrete antigen-presenting vesicles. J Exp Med 183, 1161–1172. 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muntasell A, Berger AC, and Roche PA (2007). T cell-induced secretion of MHC class II-peptide complexes on B cell exosomes. EMBO J 26, 4263–4272. 10.1038/sj.emboj.7601842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, Papworth GD, Zahorchak AF, Logar AJ, Wang Z, Watkins SC, et al. (2004). Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood 104, 3257–3266. 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- 54.Mallegol J, Van Niel G, Lebreton C, Lepelletier Y, Candalh C, Dugave C, Heath JK, Raposo G, Cerf-Bensussan N, and Heyman M. (2007). T84-intestinal epithelial exosomes bear MHC class II/peptide complexes potentiating antigen presentation by dendritic cells. Gastroenterology 132, 1866–1876. 10.1053/j.gastro.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 55.Fu C, Peng P, Loschko J, Feng L, Pham P, Cui W, Lee KP, Krug AB, and Jiang A. (2020). Plasmacytoid dendritic cells cross-prime naive CD8 T cells by transferring antigen to conventional dendritic cells through exosomes. Proc Natl Acad Sci U S A 117, 23730–23741. 10.1073/pnas.2002345117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marino J, Babiker-Mohamed MH, Crosby-Bertorini P, Paster JT, LeGuern C, Germana S, Abdi R, Uehara M, Kim JI, Markmann JF, et al. (2016). Donor exosomes rather than passenger leukocytes initiate alloreactive T cell responses after transplantation. Sci Immunol 1. 10.1126/sciimmunol.aaf8759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alexander M, Ramstead AG, Bauer KM, Lee SH, Runtsch MC, Wallace J, Huffaker TB, Larsen DK, Tolmachova T, Seabra MC, et al. (2017). Rab27-Dependent Exosome Production Inhibits Chronic Inflammation and Enables Acute Responses to Inflammatory Stimuli. J Immunol 199, 3559–3570. 10.4049/jimmunol.1700904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wiley RD, and Gummuluru S. (2006). Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection. Proc Natl Acad Sci U S A 103, 738–743. 10.1073/pnas.0507995103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Santiana M, Ghosh S, Ho BA, Rajasekaran V, Du WL, Mutsafi Y, De Jesus-Diaz DA, Sosnovtsev SV, Levenson EA, Parra GI, et al. (2018). Vesicle-Cloaked Virus Clusters Are Optimal Units for Inter-organismal Viral Transmission. Cell Host Microbe 24, 208–220 e208. 10.1016/j.chom.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Regev-Rudzki N, Wilson DW, Carvalho TG, Sisquella X, Coleman BM, Rug M, Bursac D, Angrisano F, Gee M, Hill AF, et al. (2013). Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell 153, 1120–1133. 10.1016/j.cell.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 61.Fevrier B, Vilette D, Archer F, Loew D, Faigle W, Vidal M, Laude H, and Raposo G. (2004). Cells release prions in association with exosomes. Proc Natl Acad Sci U S A 101, 9683–9688. 10.1073/pnas.0308413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen YH, Du W, Hagemeijer MC, Takvorian PM, Pau C, Cali A, Brantner CA, Stempinski ES, Connelly PS, Ma HC, et al. (2015). Phosphatidylserine vesicles enable efficient en bloc transmission of enteroviruses. Cell 160, 619–630. 10.1016/j.cell.2015.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abrami L, Brandi L, Moayeri M, Brown MJ, Krantz BA, Leppla SH, and van der Goot FG (2013). Hijacking multivesicular bodies enables long-term and exosome-mediated long-distance action of anthrax toxin. Cell Rep 5, 986–996. 10.1016/j.celrep.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sullivan JA, Tomita Y, Jankowska-Gan E, Lema DA, Arvedson MP, Nair A, Bracamonte-Baran W, Zhou Y, Meyer KK, Zhong W, et al. (2020). Treg-Cell-Derived IL-35-Coated Extracellular Vesicles Promote Infectious Tolerance. Cell Rep 30, 1039–1051 e1035. 10.1016/j.celrep.2019.12.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim YS, Lee WH, Choi EJ, Choi JP, Heo YJ, Gho YS, Jee YK, Oh YM, and Kim YK (2015). Extracellular vesicles derived from Gram-negative bacteria, such as Escherichia coli, induce emphysema mainly via IL-17A-mediated neutrophilic inflammation. J Immunol 194, 3361–3368. 10.4049/jimmunol.1402268. [DOI] [PubMed] [Google Scholar]
- 66.Teng Y, Ren Y, Sayed M, Hu X, Lei C, Kumar A, Hutchins E, Mu J, Deng Z, Luo C, et al. (2018). Plant-Derived Exosomal MicroRNAs Shape the Gut Microbiota. Cell Host Microbe 24, 637–652 e638. 10.1016/j.chom.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang X, Deeke SA, Ning Z, Starr AE, Butcher J, Li J, Mayne J, Cheng K, Liao B, Li L, et al. (2018). Metaproteomics reveals associations between microbiome and intestinal extracellular vesicle proteins in pediatric inflammatory bowel disease. Nat Commun 9, 2873. 10.1038/s41467-018-05357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hendricks MR, Lane S, Melvin JA, Ouyang Y, Stolz DB, Williams JV, Sadovsky Y, and Bomberger JM (2021). Extracellular vesicles promote transkingdom nutrient transfer during viral-bacterial co-infection. Cell Rep 34, 108672. 10.1016/j.celrep.2020.108672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cianciaruso C, Phelps EA, Pasquier M, Hamelin R, Demurtas D, Alibashe Ahmed M, Piemonti L, Hirosue S, Swartz MA, De Palma M, et al. (2017). Primary Human and Rat beta-Cells Release the Intracellular Autoantigens GAD65, IA-2, and Proinsulin in Exosomes Together With Cytokine-Induced Enhancers of Immunity. Diabetes 66, 460–473. 10.2337/db16-0671. [DOI] [PubMed] [Google Scholar]
- 70.Guay C, Menoud V, Rome S, and Regazzi R. (2015). Horizontal transfer of exosomal microRNAs transduce apoptotic signals between pancreatic beta-cells. Cell Commun Signal 13, 17. 10.1186/s12964-015-0097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Javeed N, Her TK, Brown MR, Vanderboom P, Rakshit K, Egan AM, Vella A, Lanza I, and Matveyenko AV (2021). Pro-inflammatory beta cell small extracellular vesicles induce beta cell failure through activation of the CXCL10/CXCR3 axis in diabetes. Cell Rep 36, 109613. 10.1016/j.celrep.2021.109613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rutman AK, Negi S, Gasparrini M, Hasilo CP, Tchervenkov J, and Paraskevas S. (2018). Immune Response to Extracellular Vesicles From Human Islets of Langerhans in Patients With Type 1 Diabetes. Endocrinology 159, 3834–3847. 10.1210/en.2018-00649. [DOI] [PubMed] [Google Scholar]
- 73.Bonjoch L, Casas V, Carrascal M, and Closa D. (2016). Involvement of exosomes in lung inflammation associated with experimental acute pancreatitis. The Journal of Pathology 240, 235–245. 10.1002/path.4771. [DOI] [PubMed] [Google Scholar]
- 74.Carrascal M, Areny-Balaguero A, de-Madaria E, Cardenas-Jaen K, Garcia-Rayado G, Rivera R, Martin Mateos RM, Pascual-Moreno I, Gironella M, Abian J, and Closa D. (2022). Inflammatory capacity of exosomes released in the early stages of acute pancreatitis predicts the severity of the disease. J Pathol 256, 83–92. 10.1002/path.5811. [DOI] [PubMed] [Google Scholar]
- 75.Choi HI, Choi JP, Seo J, Kim BJ, Rho M, Han JK, and Kim JG (2017). Helicobacter pylori-derived extracellular vesicles increased in the gastric juices of gastric adenocarcinoma patients and induced inflammation mainly via specific targeting of gastric epithelial cells. Exp Mol Med 49, e330. 10.1038/emm.2017.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun B, and Karin M. (2012). Obesity, inflammation, and liver cancer. J Hepatol 56, 704713. 10.1016/j.jhep.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ji Y, Luo Z, Gao H, Dos Reis FCG, Bandyopadhyay G, Jin Z, Manda KA, Isaac R, Yang M, Fu W, et al. (2021). Hepatocyte-derived exosomes from early onset obese mice promote insulin sensitivity through miR-3075. Nat Metab 3, 1163–1174. 10.1038/s42255-021-00444-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ying W, Gao H, Dos Reis FCG, Bandyopadhyay G, Ofrecio JM, Luo Z, Ji Y, Jin Z, Ly C, and Olefsky JM (2021). MiR-690, an exosomal-derived miRNA from M2-polarized macrophages, improves insulin sensitivity in obese mice. Cell Metab 33, 781–790 e785. 10.1016/j.cmet.2020.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Michelotti GA, Machado MV, and Diehl AM (2013). NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol 10, 656–665. 10.1038/nrgastro.2013.183. [DOI] [PubMed] [Google Scholar]
- 80.Gao H, Jin Z, Bandyopadhyay G, Cunha ERK, Liu X, Zhao H, Zhang D, Jouihan H, Pourshahian S, Kisseleva T, et al. (2022). MiR-690 treatment causes decreased fibrosis and steatosis and restores specific Kupffer cell functions in NASH. Cell Metab 34, 978–990 e974. 10.1016/j.cmet.2022.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, and Hotamisligil GS (2006). Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313, 1137–1140. 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dasgupta D, Nakao Y, Mauer AS, Thompson JM, Sehrawat TS, Liao CY, Krishnan A, Lucien F, Guo Q, Liu M, et al. (2020). IRE1A Stimulates Hepatocyte-Derived Extracellular Vesicles That Promote Inflammation in Mice With Steatohepatitis. Gastroenterology 159, 1487–1503 e1417. 10.1053/j.gastro.2020.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guo Q, Furuta K, Lucien F, Gutierrez Sanchez LH, Hirsova P, Krishnan A, Kabashima A, Pavelko KD, Madden B, Alhuwaish H, et al. (2019). Integrin beta(1)-enriched extracellular vesicles mediate monocyte adhesion and promote liver inflammation in murine NASH. J Hepatol 71, 1193–1205. 10.1016/j.jhep.2019.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Taylor J, Azimi I, Monteith G, and Bebawy M. (2020). Ca(2+) mediates extracellular vesicle biogenesis through alternate pathways in malignancy. J Extracell Vesicles 9, 1734326. 10.1080/20013078.2020.1734326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yu X, Harris SL, and Levine AJ (2006). The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res 66, 4795–4801. 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- 86.Kilinc S, Paisner R, Camarda R, Gupta S, Momcilovic O, Kohnz RA, Avsaroglu B, L’Etoile ND, Perera RM, Nomura DK, and Goga A. (2021). Oncogene-regulated release of extracellular vesicles. Dev Cell 56, 1989–2006 e1986. 10.1016/j.devcel.2021.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Choi D, Montermini L, Meehan B, Lazaris A, Metrakos P, and Rak J. (2021). Oncogenic RAS drives the CRAF-dependent extracellular vesicle uptake mechanism coupled with metastasis. J Extracell Vesicles 10, e12091. 10.1002/jev2.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, Lee JJ, and Kalluri R. (2017). Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 546, 498–503. 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nakase I, Kobayashi NB, Takatani-Nakase T, and Yoshida T. (2015). Active macropinocytosis induction by stimulation of epidermal growth factor receptor and oncogenic Ras expression potentiates cellular uptake efficacy of exosomes. Sci Rep 5, 10300. 10.1038/srep10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee TH, Chennakrishnaiah S, Audemard E, Montermini L, Meehan B, and Rak J. (2014). Oncogenic ras-driven cancer cell vesiculation leads to emission of double-stranded DNA capable of interacting with target cells. Biochem Biophys Res Commun 451, 295–301. 10.1016/j.bbrc.2014.07.109. [DOI] [PubMed] [Google Scholar]
- 91.McKenzie AJ, Hoshino D, Hong NH, Cha DJ, Franklin JL, Coffey RJ, Patton JG, and Weaver AM (2016). KRAS-MEK Signaling Controls Ago2 Sorting into Exosomes. Cell Rep 15, 978–987. 10.1016/j.celrep.2016.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lin S, and Gregory RI (2015). MicroRNA biogenesis pathways in cancer. Nat Rev Cancer 15, 321–333. 10.1038/nrc3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Melo SA, Sugimoto H, O’Connell JT, Kato N, Villanueva A, Vidal A, Qiu L, Vitkin E, Perelman LT, Melo CA, et al. (2014). Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell 26, 707–721. 10.1016/j.ccell.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hinzman CP, Singh B, Bansal S, Li Y, Iliuk A, Girgis M, Herremans KM, Trevino JG, Singh VK, Banerjee PP, and Cheema AK (2022). A multi-omics approach identifies pancreatic cancer cell extracellular vesicles as mediators of the unfolded protein response in normal pancreatic epithelial cells. J Extracell Vesicles 11, e12232. 10.1002/jev2.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Putz U, Howitt J, Doan A, Goh CP, Low LH, Silke J, and Tan SS (2012). The tumor suppressor PTEN is exported in exosomes and has phosphatase activity in recipient cells. Sci Signal 5, ra70. 10.1126/scisignal.2003084. [DOI] [PubMed] [Google Scholar]
- 96.Wu CH, Li J, Li L, Sun J, Fabbri M, Wayne AS, Seeger RC, and Jong AY (2019). Extracellular vesicles derived from natural killer cells use multiple cytotoxic proteins and killing mechanisms to target cancer cells. J Extracell Vesicles 8, 1588538. 10.1080/20013078.2019.1588538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vulpis E, Loconte L, Peri A, Molfetta R, Caracciolo G, Masuelli L, Tomaipitinca L, Peruzzi G, Petillo S, Petrucci MT, et al. (2022). Impact on NK cell functions of acute versus chronic exposure to extracellular vesicle-associated MICA: Dual role in cancer immunosurveillance. J Extracell Vesicles 11, e12176. 10.1002/jev2.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, Yu Z, Yang J, Wang B, Sun H, et al. (2018). Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 560, 382–386. 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Poggio M, Hu T, Pai CC, Chu B, Belair CD, Chang A, Montabana E, Lang UE, Fu Q, Fong L, and Blelloch R. (2019). Suppression of Exosomal PD-L1 Induces Systemic Anti-tumor Immunity and Memory. Cell 177, 414–427 e413. 10.1016/j.cell.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Salimu J, Webber J, Gurney M, Al-Taei S, Clayton A, and Tabi Z. (2017). Dominant immunosuppression of dendritic cell function by prostate-cancer-derived exosomes. J Extracell Vesicles 6, 1368823. 10.1080/20013078.2017.1368823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yin X, Zeng W, Wu B, Wang L, Wang Z, Tian H, Wang L, Jiang Y, Clay R, Wei X, et al. (2020). PPARalpha Inhibition Overcomes Tumor-Derived Exosomal Lipid-Induced Dendritic Cell Dysfunction. Cell Rep 33, 108278. 10.1016/j.celrep.2020.108278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zeng Q, Saghafinia S, Chryplewicz A, Fournier N, Christe L, Xie YQ, Guillot J, Yucel S, Li P, Galvan JA, et al. (2022). Aberrant hyperexpression of the RNA binding protein FMRP in tumors mediates immune evasion. Science 378, eabl7207. 10.1126/science.abl7207. [DOI] [PubMed] [Google Scholar]
- 103.Basso D, Gnatta E, Padoan A, Fogar P, Furlanello S, Aita A, Bozzato D, Zambon CF, Arrigoni G, Frasson C, et al. (2017). PDAC-derived exosomes enrich the microenvironment in MDSCs in a SMAD4-dependent manner through a new calcium related axis. Oncotarget 8, 84928–84944. 10.18632/oncotarget.20863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pucci F, Garris C, Lai CP, Newton A, Pfirschke C, Engblom C, Alvarez D, Sprachman M, Evavold C, Magnuson A, et al. (2016). SCS macrophages suppress melanoma by restricting tumor-derived vesicle-B cell interactions. Science 352, 242–246. 10.1126/science.aaf1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dror S, Sander L, Schwartz H, Sheinboim D, Barzilai A, Dishon Y, Apcher S, Golan T, Greenberger S, Barshack I, et al. (2016). Melanoma miRNA trafficking controls tumour primary niche formation. Nat Cell Biol 18, 1006–1017. 10.1038/ncb3399. [DOI] [PubMed] [Google Scholar]
- 106.Ma S, McGuire MH, Mangala LS, Lee S, Stur E, Hu W, Bayraktar E, Villar-Prados A, Ivan C, Wu SY, et al. (2021). Gain-of-function p53 protein transferred via small extracellular vesicles promotes conversion of fibroblasts to a cancer-associated phenotype. Cell Rep 34, 108726. 10.1016/j.celrep.2021.108726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vu LT, Peng B, Zhang DX, Ma V, Mathey-Andrews CA, Lam CK, Kiomourtzis T, Jin J, McReynolds L, Huang L, et al. (2019). Tumor-secreted extracellular vesicles promote the activation of cancer-associated fibroblasts via the transfer of microRNA-125b. J Extracell Vesicles 8, 1599680. 10.1080/20013078.2019.1599680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Webber J, Steadman R, Mason MD, Tabi Z, and Clayton A. (2010). Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res 70, 9621–9630. 10.1158/0008-5472.CAN-10-1722. [DOI] [PubMed] [Google Scholar]
- 109.Nabet BY, Qiu Y, Shabason JE, Wu TJ, Yoon T, Kim BC, Benci JL, DeMichele AM, Tchou J, Marcotrigiano J, and Minn AJ (2017). Exosome RNA Unshielding Couples Stromal Activation to Pattern Recognition Receptor Signaling in Cancer. Cell 170, 352–366 e313. 10.1016/j.cell.2017.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Donnarumma E, Fiore D, Nappa M, Roscigno G, Adamo A, Iaboni M, Russo V, Affinito A, Puoti I, Quintavalle C, et al. (2017). Cancer-associated fibroblasts release exosomal microRNAs that dictate an aggressive phenotype in breast cancer. Oncotarget 8, 19592–19608. 10.18632/oncotarget.14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nigri J, Leca J, Tubiana SS, Finetti P, Guillaumond F, Martinez S, Lac S, Iovanna JL, Audebert S, Camoin L, et al. (2022). CD9 mediates the uptake of extracellular vesicles from cancer-associated fibroblasts that promote pancreatic cancer cell aggressiveness. Sci Signal 15, eabg8191. 10.1126/scisignal.abg8191. [DOI] [PubMed] [Google Scholar]
- 112.Han S, Gonzalo DH, Feely M, Rinaldi C, Belsare S, Zhai H, Kalra K, Gerber MH, Forsmark CE, and Hughes SJ (2018). Stroma-derived extracellular vesicles deliver tumor-suppressive miRNAs to pancreatic cancer cells. Oncotarget 9, 5764–5777. 10.18632/oncotarget.23532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen Y, McAndrews KM, and Kalluri R. (2021). Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat Rev Clin Oncol 18, 792–804. 10.1038/s41571-021-00546-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hoshino D, Kirkbride KC, Costello K, Clark ES, Sinha S, Grega-Larson N, Tyska MJ, and Weaver AM (2013). Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep 5, 1159–1168. 10.1016/j.celrep.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sung BH, Ketova T, Hoshino D, Zijlstra A, and Weaver AM (2015). Directional cell movement through tissues is controlled by exosome secretion. Nat Commun 6, 7164. 10.1038/ncomms8164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zomer A, Maynard C, Verweij FJ, Kamermans A, Schafer R, Beerling E, Schiffelers RM, de Wit E, Berenguer J, Ellenbroek SIJ, et al. (2015). In Vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell 161, 1046–1057. 10.1016/j.cell.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lazaro-Ibanez E, Neuvonen M, Takatalo M, Thanigai Arasu U, Capasso C, Cerullo V, Rhim JS, Rilla K, Yliperttula M, and Siljander PR (2017). Metastatic state of parent cells influences the uptake and functionality of prostate cancer cell-derived extracellular vesicles. J Extracell Vesicles 6, 1354645. 10.1080/20013078.2017.1354645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hyenne V, Ghoroghi S, Collot M, Bons J, Follain G, Harlepp S, Mary B, Bauer J, Mercier L, Busnelli I, et al. (2019). Studying the Fate of Tumor Extracellular Vesicles at High Spatiotemporal Resolution Using the Zebrafish Embryo. Dev Cell 48, 554–572 e557. 10.1016/j.devcel.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 119.Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos G, Ghajar C, et al. (2012). Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 18, 883–891. 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, et al. (2015). Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol 17, 816–826. 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hood JL, San RS, and Wickline SA (2011). Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res 71, 3792–3801. 10.1158/0008-5472.CAN-10-4455. [DOI] [PubMed] [Google Scholar]
- 122.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, et al. (2015). Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335. 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kang M, Jordan V, Blenkiron C, and Chamley LW (2021). Biodistribution of extracellular vesicles following administration into animals: A systematic review. J Extracell Vesicles 10, e12085. 10.1002/jev2.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Plebanek MP, Angeloni NL, Vinokour E, Li J, Henkin A, Martinez-Marin D, Filleur S, Bhowmick R, Henkin J, Miller SD, et al. (2017). Pre-metastatic cancer exosomes induce immune surveillance by patrolling monocytes at the metastatic niche. Nat Commun 8, 1319. 10.1038/s41467-017-01433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McKiernan J, Donovan MJ, O’Neill V, Bentink S, Noerholm M, Belzer S, Skog J, Kattan MW, Partin A, Andriole G, et al. (2016). A Novel Urine Exosome Gene Expression Assay to Predict High-grade Prostate Cancer at Initial Biopsy. JAMA Oncol 2, 882–889. 10.1001/jamaoncol.2016.0097. [DOI] [PubMed] [Google Scholar]
- 126.Donovan MJ, Noerholm M, Bentink S, Belzer S, Skog J, O’Neill V, Cochran JS, and Brown GA (2015). A molecular signature of PCA3 and ERG exosomal RNA from non-DRE urine is predictive of initial prostate biopsy result. Prostate Cancer Prostatic Dis 18, 370–375. 10.1038/pcan.2015.40. [DOI] [PubMed] [Google Scholar]
- 127.Almeida A, Gabriel M, Firlej V, Martin-Jaular L, Lejars M, Cipolla R, Petit F, Vogt N, San-Roman M, Dingli F, et al. (2022). Urinary extracellular vesicles contain mature transcriptome enriched in circular and long noncoding RNAs with functional significance in prostate cancer. J Extracell Vesicles 11, e12210. 10.1002/jev2.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Clos-Garcia M, Loizaga-Iriarte A, Zuniga-Garcia P, Sanchez-Mosquera P, Rosa Cortazar A, Gonzalez E, Torrano V, Alonso C, Perez-Cormenzana M, Ugalde-Olano A, et al. (2018). Metabolic alterations in urine extracellular vesicles are associated to prostate cancer pathogenesis and progression. J Extracell Vesicles 7, 1470442. 10.1080/20013078.2018.1470442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Northrop-Albrecht EJ, Taylor WR, Huang BQ, Kisiel JB, and Lucien F. (2022). Assessment of extracellular vesicle isolation methods from human stool supernatant. J Extracell Vesicles 11, e12208. 10.1002/jev2.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Allenson K, Castillo J, San Lucas FA, Scelo G, Kim DU, Bernard V, Davis G, Kumar T, Katz M, Overman MJ, et al. (2017). High prevalence of mutant KRAS in circulating exosome-derived DNA from early-stage pancreatic cancer patients. Ann Oncol 28, 741–747. 10.1093/annonc/mdx004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kahlert C, Melo SA, Protopopov A, Tang J, Seth S, Koch M, Zhang J, Weitz J, Chin L, Futreal A, and Kalluri R. (2014). Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J Biol Chem 289, 3869–3875. 10.1074/jbc.C113.532267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang S, Che SP, Kurywchak P, Tavormina JL, Gansmo LB, Correa de Sampaio P, Tachezy M, Bockhorn M, Gebauer F, Haltom AR, et al. (2017). Detection of mutant KRAS and TP53 DNA in circulating exosomes from healthy individuals and patients with pancreatic cancer. Cancer Biol Ther 18, 158–165. 10.1080/15384047.2017.1281499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, et al. (2015). Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 523, 177–182. 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hoshino A, Kim HS, Bojmar L, Gyan KE, Cioffi M, Hernandez J, Zambirinis CP, Rodrigues G, Molina H, Heissel S, et al. (2020). Extracellular Vesicle and Particle Biomarkers Define Multiple Human Cancers. Cell 182, 1044–1061 e1018. 10.1016/j.cell.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wu X, Zhao H, Natalia A, Lim CZJ, Ho NRY, Ong CJ, Teo MCC, So JBY, and Shao H. (2020). Exosome-templated nanoplasmonics for multiparametric molecular profiling. Sci Adv 6, eaba2556. 10.1126/sciadv.aba2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang P, Wu X, Gardashova G, Yang Y, Zhang Y, Xu L, and Zeng Y. (2020). Molecular and functional extracellular vesicle analysis using nanopatterned microchips monitors tumor progression and metastasis. Sci Transl Med 12. 10.1126/scitranslmed.aaz2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cordonnier M, Nardin C, Chanteloup G, Derangere V, Algros MP, Arnould L, Garrido C, Aubin F, and Gobbo J. (2020). Tracking the evolution of circulating exosomal-PD-L1 to monitor melanoma patients. J Extracell Vesicles 9, 1710899. 10.1080/20013078.2019.1710899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shi A, Kasumova GG, Michaud WA, Cintolo-Gonzalez J, Diaz-Martinez M, Ohmura J, Mehta A, Chien I, Frederick DT, Cohen S, et al. (2020). Plasma-derived extracellular vesicle analysis and deconvolution enable prediction and tracking of melanoma checkpoint blockade outcome. Sci Adv 6. 10.1126/sciadv.abb3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cornell L, Wander SA, Visal T, Wagle N, and Shapiro GI (2019). MicroRNA-Mediated Suppression of the TGF-beta Pathway Confers Transmissible and Reversible CDK4/6 Inhibitor Resistance. Cell Rep 26, 2667–2680 e2667. 10.1016/j.celrep.2019.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Park M, Kim JW, Kim KM, Kang S, Kim W, Kim JK, Cho Y, Lee H, Baek MC, Bae JH, et al. (2021). Circulating Small Extracellular Vesicles Activate TYRO3 to Drive Cancer Metastasis and Chemoresistance. Cancer Res 81, 3539–3553. 10.1158/0008-5472.CAN-20-3320. [DOI] [PubMed] [Google Scholar]
- 141.Kreger BT, Johansen ER, Cerione RA, and Antonyak MA (2016). The Enrichment of Survivin in Exosomes from Breast Cancer Cells Treated with Paclitaxel Promotes Cell Survival and Chemoresistance. Cancers (Basel) 8. 10.3390/cancers8120111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ma X, Chen Z, Hua D, He D, Wang L, Zhang P, Wang J, Cai Y, Gao C, Zhang X, et al. (2014). Essential role for TrpC5-containing extracellular vesicles in breast cancer with chemotherapeutic resistance. Proc Natl Acad Sci U S A 111, 6389–6394. 10.1073/pnas.1400272111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ma S, Mangala LS, Hu W, Bayaktar E, Yokoi A, Hu W, Pradeep S, Lee S, Piehowski PD, Villar-Prados A, et al. (2021). CD63-mediated cloaking of VEGF in small extracellular vesicles contributes to anti-VEGF therapy resistance. Cell Rep 36, 109549. 10.1016/j.celrep.2021.109549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Qu L, Ding J, Chen C, Wu ZJ, Liu B, Gao Y, Chen W, Liu F, Sun W, Li XF, et al. (2016). Exosome-Transmitted lncARSR Promotes Sunitinib Resistance in Renal Cancer by Acting as a Competing Endogenous RNA. Cancer Cell 29, 653–668. 10.1016/j.ccell.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 145.Pavlyukov MS, Yu H, Bastola S, Minata M, Shender VO, Lee Y, Zhang S, Wang J, Komarova S, Wang J, et al. (2018). Apoptotic Cell-Derived Extracellular Vesicles Promote Malignancy of Glioblastoma Via Intercellular Transfer of Splicing Factors. Cancer Cell 34, 119–135 e110. 10.1016/j.ccell.2018.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Boelens MC, Wu TJ, Nabet BY, Xu B, Qiu Y, Yoon T, Azzam DJ, Twyman-Saint Victor C, Wiemann BZ, Ishwaran H, et al. (2014). Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell 159, 499–513. 10.1016/j.cell.2014.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sansone P, Savini C, Kurelac I, Chang Q, Amato LB, Strillacci A, Stepanova A, Iommarini L, Mastroleo C, Daly L, et al. (2017). Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc Natl Acad Sci U S A 114, E9066–E9075. 10.1073/pnas.1704862114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yuan X, Duan Y, Xiao Y, Sun K, Qi Y, Zhang Y, Ahmed Z, Moiani D, Yao J, Li H, et al. (2022). Vitamin E Enhances Cancer Immunotherapy by Reinvigorating Dendritic Cells via Targeting Checkpoint SHP1. Cancer Discov 12, 1742–1759. 10.1158/2159-8290.CD-21-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Majzner RG, and Mackall CL (2019). Clinical lessons learned from the first leg of the CAR T cell journey. Nat Med 25, 1341–1355. 10.1038/s41591-019-0564-6. [DOI] [PubMed] [Google Scholar]
- 150.Johnson LR, Lee DY, Eacret JS, Ye D, June CH, and Minn AJ (2021). The immunostimulatory RNA RN7SL1 enables CAR-T cells to enhance autonomous and endogenous immune function. Cell 184, 4981–4995 e4914. 10.1016/j.cell.2021.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mendt M, Kamerkar S, Sugimoto H, McAndrews KM, Wu CC, Gagea M, Yang S, Blanko EVR, Peng Q, Ma X, et al. (2018). Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight 3. 10.1172/jci.insight.99263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, and Frangioni JV (2007). Renal clearance of quantum dots. Nat Biotechnol 25, 1165–1170. 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Saari H, Lazaro-Ibanez E, Viitala T, Vuorimaa-Laukkanen E, Siljander P, and Yliperttula M. (2015). Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. J Control Release 220, 727–737. 10.1016/j.jconrel.2015.09.031. [DOI] [PubMed] [Google Scholar]
- 154.Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, Inskoe E, Piroyan A, Sokolsky M, Okolie O, et al. (2016). Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine 12, 655–664. 10.1016/j.nano.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yong T, Zhang X, Bie N, Zhang H, Zhang X, Li F, Hakeem A, Hu J, Gan L, Santos HA, and Yang X. (2019). Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat Commun 10, 3838. 10.1038/s41467-019-11718-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li YJ, Wu JY, Wang JM, Hu XB, Cai JX, and Xiang DX (2020). Gemcitabine loaded autologous exosomes for effective and safe chemotherapy of pancreatic cancer. Acta Biomater 101, 519–530. 10.1016/j.actbio.2019.10.022. [DOI] [PubMed] [Google Scholar]
- 157.Al Faruque H, Choi ES, Kim JH, and Kim E. (2022). Enhanced effect of autologous EVs delivering paclitaxel in pancreatic cancer. J Control Release 347, 330–346. 10.1016/j.jconrel.2022.05.012. [DOI] [PubMed] [Google Scholar]
- 158.Tian F, Zhang S, Liu C, Han Z, Liu Y, Deng J, Li Y, Wu X, Cai L, Qin L, et al. (2021). Protein analysis of extracellular vesicles to monitor and predict therapeutic response in metastatic breast cancer. Nat Commun 12, 2536. 10.1038/s41467-021-22913-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhao L, Gu C, Gan Y, Shao L, Chen H, and Zhu H. (2020). Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis. J Control Release 318, 1–15. 10.1016/j.jconrel.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 160.Xu L, Faruqu FN, Lim YM, Lim KY, Liam-Or R, Walters AA, Lavender P, Fear D, Wells CM, Tzu-Wen Wang J, and Al-Jamal KT (2021). Exosome-mediated RNAi of PAK4 prolongs survival of pancreatic cancer mouse model after loco-regional treatment. Biomaterials 264, 120369. 10.1016/j.biomaterials.2020.120369. [DOI] [PubMed] [Google Scholar]
- 161.Haltom AR, Hassen WE, Hensel J, Kim J, Sugimoto H, Li B, McAndrews KM, Conner MR, Kirtley ML, Luo X, et al. (2022). Engineered exosomes targeting MYC reverse the proneural-mesenchymal transition and extend survival of glioblastoma. Extracellular Vesicle 1. 10.1016/j.vesic.2022.100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kamerkar S, Leng C, Burenkova O, Jang SC, McCoy C, Zhang K, Dooley K, Kasera S, Zi T, Siso S, et al. (2022). Exosome-mediated genetic reprogramming of tumor-associated macrophages by exoASO-STAT6 leads to potent monotherapy antitumor activity. Sci Adv 8, eabj7002. 10.1126/sciadv.abj7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.McAndrews KM, Xiao F, Chronopoulos A, LeBleu VS, Kugeratski FG, and Kalluri R. (2021). Exosome-mediated delivery of CRISPR/Cas9 for targeting of oncogenic Kras(G12D) in pancreatic cancer. Life Sci Alliance 4. 10.26508/lsa.202000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kim SM, Yang Y, Oh SJ, Hong Y, Seo M, and Jang M. (2017). Cancer-derived exosomes as a delivery platform of CRISPR/Cas9 confer cancer cell tropism-dependent targeting. J Control Release 266, 8–16. 10.1016/j.jconrel.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 165.Usman WM, Pham TC, Kwok YY, Vu LT, Ma V, Peng B, Chan YS, Wei L, Chin SM, Azad A, et al. (2018). Efficient RNA drug delivery using red blood cell extracellular vesicles. Nat Commun 9, 2359. 10.1038/s41467-018-04791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, Fujita K, Mizutani T, Ohgi T, Ochiya T, et al. (2013). Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther 21, 185–191. 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pomatto MAC, Bussolati B, D’Antico S, Ghiotto S, Tetta C, Brizzi MF, and Camussi G. (2019). Improved Loading of Plasma-Derived Extracellular Vesicles to Encapsulate Antitumor miRNAs. Mol Ther Methods Clin Dev 13, 133–144. 10.1016/j.omtm.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shi K, Fang Y, Gao S, Yang D, Bi H, Xue J, Lu A, Li Y, Ke L, Lin X, et al. (2018). Inorganic kernel - Supported asymmetric hybrid vesicles for targeting delivery of STAT3-decoy oligonucleotides to overcome anti-HER2 therapeutic resistance of BT474R. J Control Release 279, 53–68. 10.1016/j.jconrel.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 169.Sancho-Albero M, Rubio-Ruiz B, Perez-Lopez AM, Sebastian V, Martin-Duque P, Arruebo M, Santamaria J, and Unciti-Broceta A. (2019). Cancer-derived exosomes loaded with ultrathin palladium nanosheets for targeted bioorthogonal catalysis. Nat Catal 2, 864–872. 10.1038/s41929-019-0333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhou X, Miao Y, Wang Y, He S, Guo L, Mao J, Chen M, Yang Y, Zhang X, and Gan Y. (2022). Tumour-derived extracellular vesicle membrane hybrid lipid nanovesicles enhance siRNA delivery by tumour-homing and intracellular freeway transportation. J Extracell Vesicles 11, e12198. 10.1002/jev2.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhupanyn P, Ewe A, Buch T, Malek A, Rademacher P, Muller C, Reinert A, Jaimes Y, and Aigner A. (2020). Extracellular vesicle (ECV)-modified polyethylenimine (PEI) complexes for enhanced siRNA delivery in vitro and in vivo. J Control Release 319, 63–76. 10.1016/j.jconrel.2019.12.032. [DOI] [PubMed] [Google Scholar]
- 172.Liu J, Ye Z, Xiang M, Chang B, Cui J, Ji T, Zhao L, Li Q, Deng Y, Xu L, et al. (2019). Functional extracellular vesicles engineered with lipid-grafted hyaluronic acid effectively reverse cancer drug resistance. Biomaterials 223, 119475. 10.1016/j.biomaterials.2019.119475. [DOI] [PubMed] [Google Scholar]
- 173.Jang SC, Economides KD, Moniz RJ, Sia CL, Lewis N, McCoy C, Zi T, Zhang K, Harrison RA, Lim J, et al. (2021). ExoSTING, an extracellular vesicle loaded with STING agonists, promotes tumor immune surveillance. Commun Biol 4, 497. 10.1038/s42003-021-02004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.McAndrews KM, Che SPY, LeBleu VS, and Kalluri R. (2021). Effective delivery of STING agonist using exosomes suppresses tumor growth and enhances antitumor immunity. J Biol Chem 296, 100523. 10.1016/j.jbc.2021.100523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Peng B, Nguyen TM, Jayasinghe MK, Gao C, Pham TT, Vu LT, Yeo EYM, Yap G, Wang L, Goh BC, et al. (2022). Robust delivery of RIG-I agonists using extracellular vesicles for anti-cancer immunotherapy. J Extracell Vesicles 11, e12187. 10.1002/jev2.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Andre F, Chaput N, Schartz NE, Flament C, Aubert N, Bernard J, Lemonnier F, Raposo G, Escudier B, Hsu DH, et al. (2004). Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J Immunol 172, 2126–2136. 10.4049/jimmunol.172.4.2126. [DOI] [PubMed] [Google Scholar]
- 177.Wahlund CJE, Gucluler G, Hiltbrunner S, Veerman RE, Naslund TI, and Gabrielsson S. (2017). Exosomes from antigen-pulsed dendritic cells induce stronger antigen-specific immune responses than microvesicles in vivo. Sci Rep 7, 17095. 10.1038/s41598-017-16609-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Xiong X, Ke X, Wang L, Lin Y, Wang S, Yao Z, Li K, Luo Y, Liu F, Pan Y, et al. (2022). Neoantigen-based cancer vaccination using chimeric RNA-loaded dendritic cell-derived extracellular vesicles. J Extracell Vesicles 11, e12243. 10.1002/jev2.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Fu W, Lei C, Liu S, Cui Y, Wang C, Qian K, Li T, Shen Y, Fan X, Lin F, et al. (2019). CAR exosomes derived from effector CAR-T cells have potent antitumour effects and low toxicity. Nat Commun 10, 4355. 10.1038/s41467-019-12321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dooley K, McConnell RE, Xu K, Lewis ND, Haupt S, Youniss MR, Martin S, Sia CL, McCoy C, Moniz RJ, et al. (2021). A versatile platform for generating engineered extracellular vesicles with defined therapeutic properties. Mol Ther 29, 1729–1743. 10.1016/j.ymthe.2021.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Watson DC, Yung BC, Bergamaschi C, Chowdhury B, Bear J, Stellas D, Morales-Kastresana A, Jones JC, Felber BK, Chen X, and Pavlakis GN (2018). Scalable, cGMP-compatible purification of extracellular vesicles carrying bioactive human heterodimeric IL-15/lactadherin complexes. J Extracell Vesicles 7, 1442088. 10.1080/20013078.2018.1442088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Escudier B, Dorval T, Chaput N, Andre F, Caby MP, Novault S, Flament C, 1234 Leboulaire C, Borg C, Amigorena S, et al. (2005). Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J Transl Med 3, 10. 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Besse B, Charrier M, Lapierre V, Dansin E, Lantz O, Planchard D, Le Chevalier T, Livartoski A, Barlesi F, Laplanche A, et al. (2016). Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology 5, e1071008. 10.1080/2162402X.2015.1071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Dragovic RA, Gardiner C, Brooks AS, Tannetta DS, Ferguson DJ, Hole P, Carr B, Redman CW, Harris AL, Dobson PJ, et al. (2011). Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomedicine 7, 780–788. 10.1016/j.nano.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mizenko RR, Brostoff T, Rojalin T, Koster HJ, Swindell HS, Leiserowitz GS, Wang A, and Carney RP (2021). Tetraspanins are unevenly distributed across single extracellular vesicles and bias sensitivity to multiplexed cancer biomarkers. J Nanobiotechnology 19, 250. 10.1186/s12951-021-00987-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Banijamali M, Hojer P, Nagy A, Haag P, Gomero EP, Stiller C, Kaminskyy VO, Ekman S, Lewensohn R, Karlstrom AE, et al. (2022). Characterizing single extracellular vesicles by droplet barcode sequencing for protein analysis. J Extracell Vesicles 11, e12277. 10.1002/jev2.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ko J, Wang Y, Sheng K, Weitz DA, and Weissleder R. (2021). Sequencing-Based Protein Analysis of Single Extracellular Vesicles. ACS Nano 15, 5631–5638. 10.1021/acsnano.1c00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhu X, Badawi M, Pomeroy S, Sutaria DS, Xie Z, Baek A, Jiang J, Elgamal OA, Mo X, Perle K, et al. (2017). Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J Extracell Vesicles 6, 1324730. 10.1080/20013078.2017.1324730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Cheng Q, Shi X, Han M, Smbatyan G, Lenz HJ, and Zhang Y. (2018). Reprogramming Exosomes as Nanoscale Controllers of Cellular Immunity. J Am Chem Soc 140, 16413–16417. 10.1021/jacs.8b10047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wang Z, Popowski KD, Zhu D, de Juan Abad BL, Wang X, Liu M, Lutz H, De Naeyer N, DeMarco CT, Denny TN, et al. (2022). Exosomes decorated with a recombinant SARS-CoV-2 receptor-binding domain as an inhalable COVID-19 vaccine. Nat Biomed Eng 6, 791–805. 10.1038/s41551-022-00902-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.