Fig. 3 |. Scope of nucleophiles and electrophiles that participate in defluorinative alkylation reactions and transformations of the products.
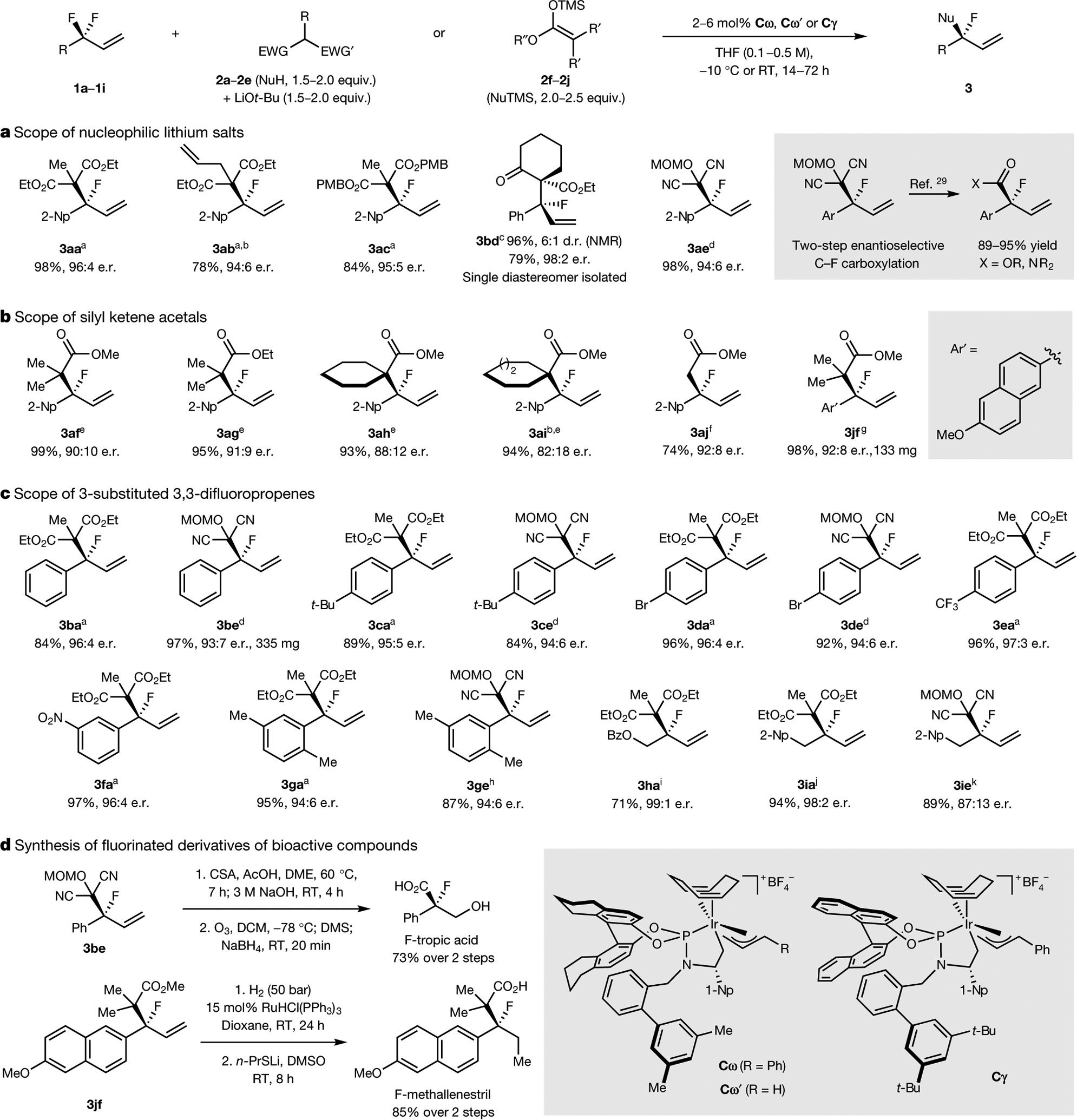
a, Nucleophilic lithium salts in defluorinative alkylation reactions. b, Silyl ketene acetals in defluorinative alkylation reactions. c, 3-Substituted 3,3-difluoropropenes in defluorinative alkylation reactions. d, Synthesis of fluorinated derivatives of medicinally active compounds from the substitution products. Isolated yields reported unless otherwise noted. a2 mol% Cω, −10 °C, 72 h. b96 h. c5 mol% Cω’, 0 °C, 72 h. d2 mol% Cω’, 5 equiv. LiBr, RT, 24 h. e6 mol% Cγ, 5 mol% NaCMe(CO2Et)2, RT, 48 h. ftert-Butyl dimethyl silyl ketene acetal, 6 mol% Cγ, RT, 53 h. g2 mol% TMSOTf, 4 mol% Cγ, dioxane, RT, 40 h. h5 mol% Cω’, 5 equiv. LiBr, RT, 48 h. i5 mol% Cω, 65 °C, 96 h. j2 mol% Cω, 3 equiv. Ba(OTf)2, RT, 19 h. k20 mol% Cω, 3 equiv. Ba(OTf)2, dioxane, RT, 24 h. EWG, electron-withdrawing group; TMS, trimethylsilyl; MOM, methoxymethyl; Bz, benzoyl; CSA, camphor sulfonic acid; DME, 1,2-dimethoxyethane; DMS, dimethylsulfide; 1-Np, 1-naphthyl; d.r., diastereometric ratio.
