Fig. 5 |. Mechanistic studies support a cation-assisted, turnover-limiting, enantiodetermining and irreversible oxidative addition from a rapidly interconverting mixture of diastereomeric olefin complexes under Curtin–Hammett control.
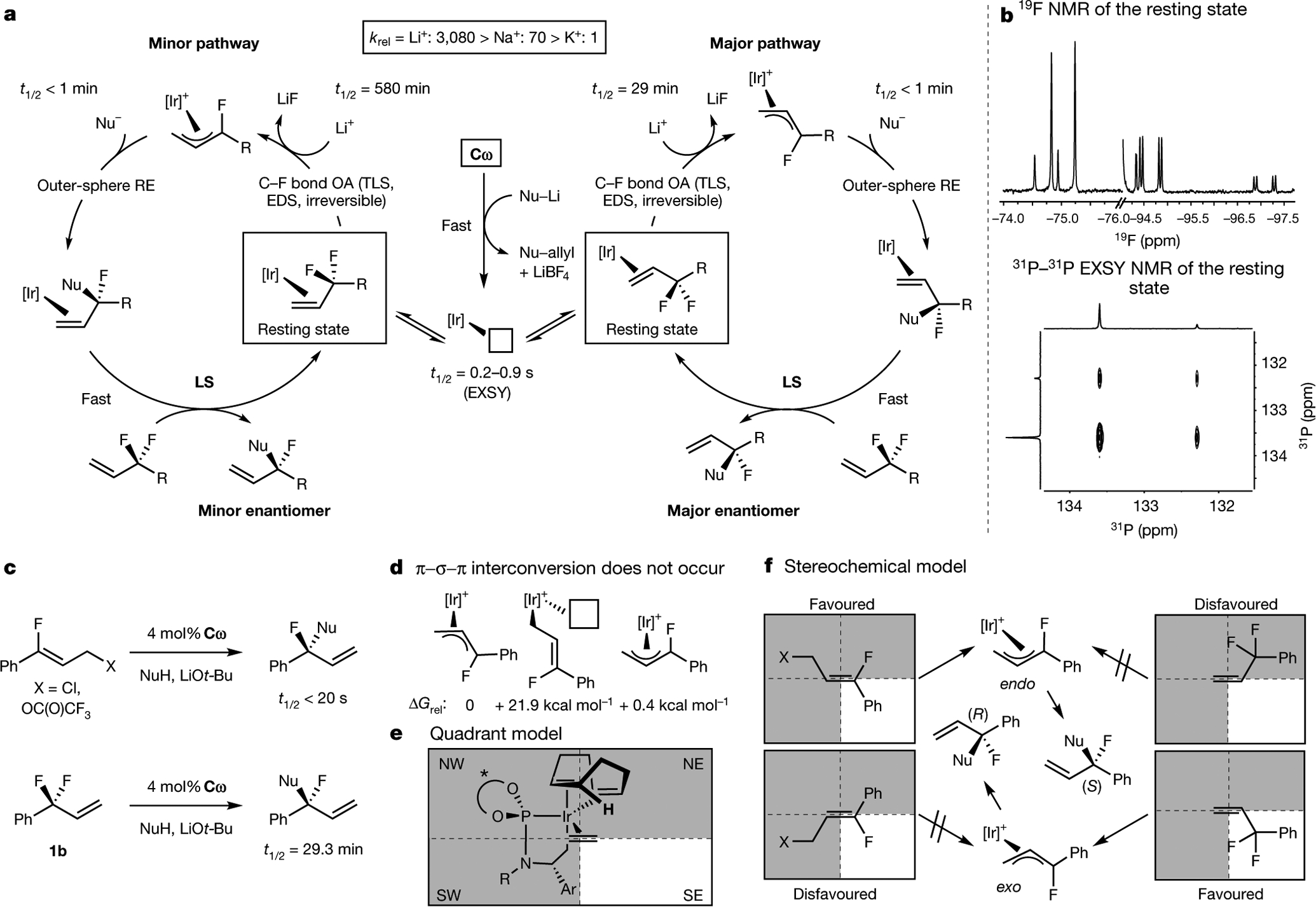
a, Proposed mechanism. b, NMR spectroscopic characterization of the catalyst resting state. c, Comparison of allylic fluoroalkylation reactions with 3-fluorocinnamyl electrophiles and 3-substituted 3,3-difluoropropenes. d, η1 allyl intermediates are too high in energy to participate. e, Quadrant diagram for Ir(i) olefin complexes. f, Stereochemical model for 3-fluorocinnamyl electrophiles and 3-substituted 3,3-difluoropropenes; see Supplementary Information for a more detailed discussion. OA, oxidative addition; RE, reductive elimination; LS, ligand substitution; TLS, turnover-limiting step; EDS, enantiodetermining step; EXSY, exchange spectroscopy; krel, relative rate constant; ΔGrel, relative Gibbs free energy.
