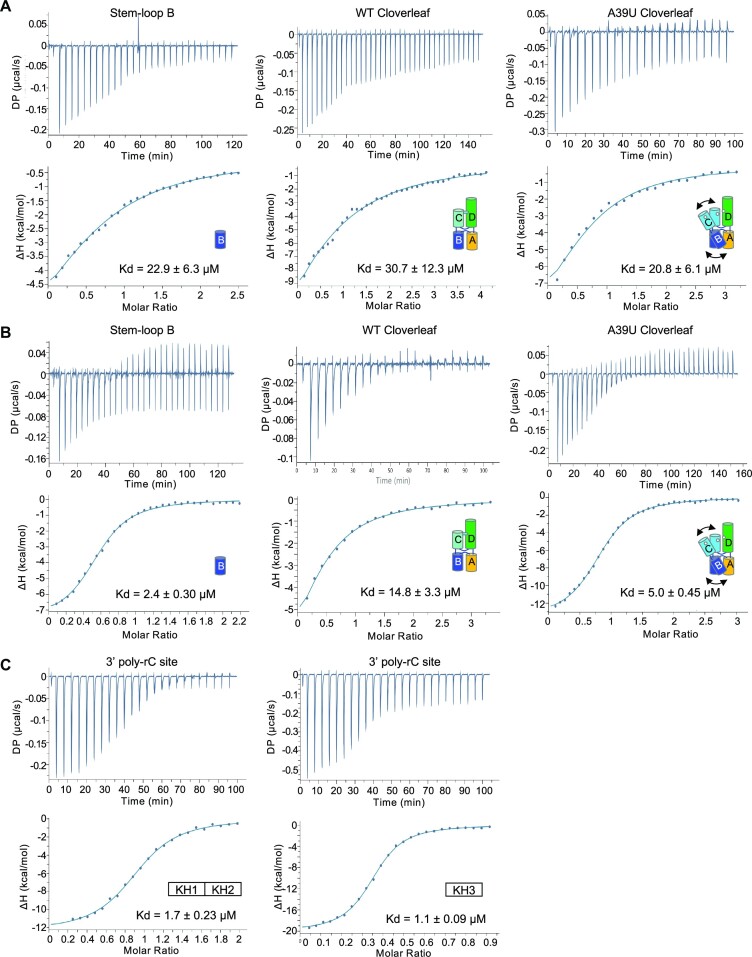
Figure 4.
Isothermal calorimetry of PCBP2 domains with poly-rC containing RNAs. (A) Interaction of PCBP2 KH1/2 domain with PV cloverleaf RNA. Isolated stem–loop B, WT cloverleaf (tRNA-CLPV) and A39U mutant cloverleaf (tRNA-CLA39U) were titrated into PCBP2 KH1/2 domain. Thermograms and fitted binding curves are shown. Data were fit with single binding-site model. Titrations were repeated at least twice. (B) Interaction of PCBP2 KH3 domain with PV cloverleaf RNA. Isolated stem–loop B, WT cloverleaf and A39U mutant cloverleaf were titrated into PCBP2 KH3 domain. The data were fit with single binding-site model. Note KH3 domain binds A39U mutant cloverleaf with higher affinity than WT cloverleaf, suggesting that stem–loop B site is not accessible in WT cloverleaf. (C) Interaction of PCBP2 domains with PV 3′poly-rC site. The linear ssRNA containing the 3′poly-rC sequence was titrated into either KH1/2 or KH3 domains. The binding affinities to the linear ssRNA are similar for both PCBP2 domains. Note that the KH3 domain binds 3′poly-rC with 2:1 (protein/RNA) stoichiometry. Summary of parameters are listed in Table S1.