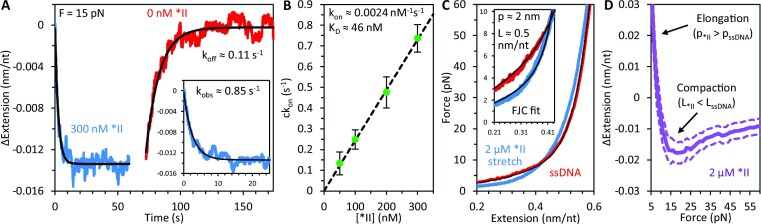
Figure 2.
Binding dynamics of *II truncate. (A) The noncooperative *II gp32 truncate exhibits single-phase binding (blue, inset shows magnified exponential fit) with significantly reduced compaction relative to WT gp32. When free *II is removed (red) the ssDNA exponentially elongates back to its original length on a 10 s timescale, consistent with full dissociation of protein. (B) The measured rate of protein binding (ckon) is directly proportional to protein concentration and linearly fit to compute the concentration-independent bimolecular on-rate and KD of *II at 15 pN. (C) When the ssDNA is slowly stretched (∼10 nm/s) in the presence of a saturating concentration (2 μM) of *II, the DNA is measurably shorter at high force (>10 pN) and longer at low force (<10 pN) due to changes in the contour and persistence lengths. The force-extension curve of the *II-saturated DNA was fit with the freely jointed chain (FJC) up to 10 pN (inset) to compute the contour (L) and persistence (p) lengths of the complex. (D) The average extension change of ssDNA as a result of *II binding is calculated at every force (1 pN increments) and plotted as a function of ssDNA tension (purple curve with dashed lines showing SEM).