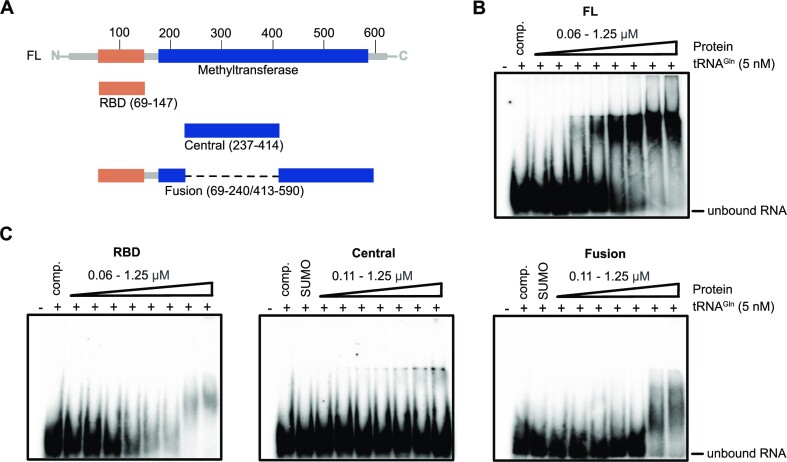
Figure 2.
hTRMT2A domains bind tRNAGln cooperatively. (A) Cartoon depicting protein fragments of hTRMT2A used for EMSAs. (B) EMSA with hTRMT2A FL shows high affinity binding to tRNAGln in the nanomolar KD range. (C) EMSAs with the hTRMT2A RBD, Central and Fusion domain display only low affinity binding to tRNAGln in the micromolar KD range. Because the SUMO-tagged Central domain was used, a SUMO-tag control was included. Binding assays were performed with 32P-labeled tRNAGln. For experiments, poly(U) competitor and labeled RNA were pre-incubated with increasing concentrations of hTRMT2A protein for 20 min. Reaction without protein served as control. Free RNA and RNA–protein complex were separated by 4% native PAGE. Each experiment was performed in triplicate.