Abstract
Biofuels are expected to play a major role in reducing carbon emissions in the aviation sector globally. Farnesane (“2,6,10-trimethyldodecane”) is a biofuel derived from the synthesized iso-paraffin route wich can be blended with jet fuel; however, the microbial behavior in farnesane/jet fuel blends remains unknown. The chemical and biological stability of blends should be investigated to ensure they meet the quality requirements for aviation fuels. This work aimed at evaluating the behavior of two fungi Hormoconis resinae (F089) and Exophiala phaeomuriformis (UFRGS Q4.2) in jet fuel, farnesane, and in 10% farnesane blend during simulated storage. Microcosms (150-mL flasks) were assembled with and without fungi containing Bushnell & Haas mineral medium for 28 days at a temperature of 20±2°C. The fungal growth (biomass), pH, surface tension, and changes in the fuel’s hydrocarbon chains were evaluated. This study revealed thatthe treatment containing H. resinae showed a biomass of 19 mg, 12 mg, and 2 mg for jet fuel, blend, and farnesane respectively. The pH was reduced from 7.2 to 4.3 observed in jet fuel treatment The degradation results showed that compounds with carbon chains between C9 and C11, in jet fuel, and blend treatments were preferably degraded. The highest biomass (70.9 mg) produced by E. phaeomuriformis was in 10% farnesane blend, after 21 days. However, no significant decrease was observed on pH and surface tension measurements across the treatments as well as on the hydrocarbons when compared to the controls. This study revealed that farnesane neither inhibited nor promoted greater growth on both microorganisms.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-023-01055-6.
Keywords: Biodeterioration, Jet fuel, Biojet fuel, Microbial contamination
Introduction
There is an increasing interest in the use of alternative fuels in the aviation sector globally due to concerns on the high carbon emissionsfrom fossil fuels [1]. Sustainable aviation fuel derived from biomass has become a potential solution to reduce carbon emissions as it can be blended with conventional aviation fuel in specific fractional volumes [2–4]. Currently, commercial civil aviation uses aviation jet fuel which requires special attention during storage due to its high susceptibility to microbial contamination. The implications of microbial growth in commercial fuels are potentially serious as microbial activity in jet fuel can cause problems related to fuel deterioration which are decreased surface tension, clogging of fuel filters, and microorganism-induced corrosion [5–10]. Blending fossil jet fuels with sustainable aviation fuel is essential to reducing carbon emissions; however, the microbial behavior and its consequences in jet fuel blends are still underexplored.
Jet fuels are petroleum products used as propellants in modern airplanes equipped with turbine engines, whether pure jets, turboprops, or turbofans. They are fractions distilled from petroleum with a boiling range that varies according to the application and brand: for commercial aircraft Jet-A1, Jet-A, and Jet-B. The most used fuel in the world is Jet-A1, with conventional aviation fuels composed of hydrocarbons: aromatics, cycloalkanes, branched alkanes, and n-alkanes. The percentage of each fraction is variable and depends on the crude oil used in the refining process [11, 12].
One of the first reports of microorganisms in aircraft fuel tanks with samples from different US Air Force bases was carried out by Bakanaukas in 1958 [13]. In Brazil, the fungus Hormoconis resinae was isolated from sediments of jet fuel tanks as reported in 1966 by Gutheil [14]. H. resinae is one of the most studied microorganisms, and it is known as one of the most deteriogenic fungus in jet fuel and diesel oil [6, 7, 9, 15–27]. It has been shown that middle distillate fuels such as aviation kerosene and diesel oil are susceptible to microbial growth since carbon chains (in the range of C10–C18) can be used as carbon source and energy by various microorganisms [5, 6, 28–30]. Moreover, the development of microorganisms and their relationship with the deterioration of fuels and/or blends with biofuels is dependent on competent microbial density, residence time, and the presence of water [8, 31, 32]. Furthermore, the presence of water provides a favorable environment for the proliferation of microorganisms in the tank, promoting the development of microbial communities on the surfaces of fuel tanks or at the fuel-water interface [5, 29, 30, 32].
The assessment of microbial contamination in aviation fuels is performed according to several established standards, and involves counting heterotrophic bacteria on plates (ASTM-D6974-16), enzyme immunosorbent assays (ASTM-D8070-16), adenosine triphosphate (ATP) test (ASTM-D7463-16), and polymerase chain reaction assay (ASTM-D7687-17) [5, 9, 23]. Although aviation jet fuel is one of the fuels worldwide that receives the most care and attention in terms of storage, it is essential to know the susceptibility to microbial deterioration of a possible blend with biojet fuel, with reference to simulated storage studies. Water may accumulate if fuel housekeeping is not followed and even small quantities of water can significantly increase the microbial growth. Any changes in chemical composition of fuels will influence their nutrient status and this may influence the rate and extent of microbial growth that may occur [33]. In Brazil, with the addition of biodiesel to diesel, a greater susceptibility of the blends (diesel/biodiesel) to biodeterioration and chemical instability was identified [34, 35]. Therefore, it is fundamental to investigate whether the addition of biofuel to commercial jet fuel will impact on its susceptibility to biodeterioration. The objective of this study was to evaluate the behavior of fungi H. resinae (F089) and E. phaeomuriformis (UFRGS Q4.2) in jet fuel and alternative jet fuel (farnesane). The biomass growth, production of metabolites, and the degradation of hydrocarbon fractions were evaluated under simulated storage using an aqueous mineral medium with jet fuel, farnesane, and the 10% farnesane blend after 21 and 28 days.
Materials and methods
Microorganisms
H. resinae (F089)
The F089 strain of the fungus H. resinae was sent to LAB-BIO UFRGS by the National Institute of Technology (INT-RJ). The strain was isolated from jet fuel. The genetic sequence of H. resinae (F089) was submitted to the GenBank Database (online tool available at http://blast.be-md.ncbi.nlm.nih.gov/) under the code EU040230.1.
Isolation and identification of yeast fungus isolated from jet fuel
The yeast fungus was prospected from a sample of jet fuel (Porto Alegre, Rio Grande do Sul, Brazil) according to ASTM D6974-09 (modified). In this procedure, 500 mL of jet fuel samples were filtered in triplicate, under aseptic conditions, using 0.22-μm nitrocellulose membrane filters (Millipore, USA). After filtering, the membranes were placed on a culture medium and incubated in an oven at 30°C for 7 days. From the grown organisms, one inoculating loop of cell material was taken and separated using a streak plate method. Single colonies were picked and enriched in a medium for purification of the isolates. For the molecular identification of the yeast fungus, Sanger sequencing was performed. The DNA used for sequencing was obtained from colonies of the fungus in potato dextrose agar medium (potato extract 4g L−1; dextrose 20g L−1; agar 15g L−1; final pH: 5.1 ± 0. 2 at 25°C) and incubated for 5 days at 30°C. For DNA extraction, the CTAB method was used [36]. The degree of purity of the extracted DNA was evaluated using the Nanodrop ND-1000 spectrophotometer (Nanodrop Lite, Thermo Scientific, Wilmington, DE, USA). From this DNA, PCR amplification of the “ITS-5.8S rRNA-ITS2” region was performed, using the primers: ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′). The polymerase chain reaction products were purified using Invitrogen’s PureLinkTM PCR purification kit. Sequencing reactions were performed using BigDye® Terminator 3.1 Cycle Sequencing Reaction Kit (Applied Biosystems, Foster City USA) according to the manufacturer’s instructions. Fragments were processed using the automatic sequencer ABI Prism 3130 Genetic Analyzer (Applied Biosystems, Foster City, CA). Generated sequences were compared by the automated system with the GenBank database (online tool available at (http://blast.be-md.ncbi.nlm.nih.gov/) to identify the percentage of similarity. The genetic sequence was deposited at NCBI GenBank (accession number MK 577887).
Inoculum preparation
The lyophilized fungus H. resinae (F089) was received from the INT. Strain hydration was performed by adding 1mL of Milli-Q® ultrapure water. H. resinae (F089) and the isolated yeast fungus were prepared as described below. Cultivation was carried out in potato dextrose agar (PDA) in several inclined tubes for 5 days, at a temperature of 28 ± 2°C. After this period, 2mL of 0.01% Tween 80 surfactant (Sigma-Aldrich) were added to the cultures to remove spores. Dilutions of spore suspensions were prepared with saline solution (distilled water with 0.85% NaCl) and counted in Neubauer chamber to obtain a final concentration of 106 spores mL−1.
Fuels
Sterilization of fuels
The fuels were sterilized by filtration using 0.22-μm nitrocellulose membrane filters (Millipore, USA) with the aid of a vacuum pump and a sterilized Büchner flask. They were packed in 1-liter amber glass bottles and stored in a refrigerator (4°C) for later use.
Jet fuel
Samples of commercial jet fuel collected from distribution tanks from an aviation company from Porto Alegre, Rio Grande do Sul, Brazil were used. Aviation fuel (jet fuel) is a petroleum derivative obtained by direct distillation with temperature from 150 to 300°C, with predominance of paraffinic hydrocarbons from 9 to 15 carbon atoms, used in aeronautics turbines. In Brazil, jet fuel is produced by Petrobras [37]. The product specifications are regulated by the National Agency for Oil, Natural Gas, and Biofuel (ANP) [38]. Samples were collected directly from storage tanks in sterile plastic bottles and taken to the laboratory. The total volume of 3 L collected was kept closed under refrigeration (4°C). Jet fuel had a clear, transparent, and water-free appearance.
Farnesane
Farnesane used (3 L) was provided by a company from São Paulo, Brazil and sent to LAB-BIO UFRGS, being kept under refrigeration (4°C) in the laboratory. Farnesane was produced using the SIP route (synthesized isoparaffins) and identified as “2,6,10-trimethyldodecane” (IUPAC). The compound “2,6,10-trimethyldodecane,” also called farnesane, was marketed by a company that primarily produces farnesene (C15H32) from a genetic modification of a yeast used in the fermentation of sugar cane [4, 39]. Farnesene can be used in products for the chemical industry or in transport fuels. To be used as a biofuel, farnesene is hydrogenated, giving rise to farnesane, which has similar characteristics to aviation kerosene [40].
10% Farnesane blend
The blend of jet fuel with 10% farnesane was prepared in the LAB-BIO laboratory with jetfuel and biojet fuel under aseptic conditions.
Preliminary biodegradability test
The preliminary biodegradability of fuels (jet fuel, farnesane, and 10% farnesane blend) using the fungi H. resinae (F089) and the isolated yeast was evaluated using 2,6-dichlorophenol-indophenol (DCPIP) [41]. For the preliminary biodegradability test of the fungi, quintuplicates were used in sterile glass flasks (20 mL) containing the aqueous phases and the fuels (jet fuel, farnesane, and 10% farnesane blend). The aqueous phase was composed of 10 mL of BH mineral medium [42] (g L−1: 1.0 KH2PO4; 1.0 K2HPO4: 1.0 NH4NO3; 0.2 MgSO4; 0.05 FeCl3; 0.02 CaCl2; pH 7.2), with the DCPIP indicator (0.25g L−1) sterilized at 121°C for 15 min. The fuel phase consisted of 500 μL of jet fuel, farnesane, and 10% farnesane blend, which was handled under sterile conditions (laminar flow chamber). The negative controlwas prepared under the same conditionsand no fungal inocula were added. For the positive control, the potato dextrose broth was used and the fungal inoculum was added. The experiment was carried out for 28 days without agitation. Fungal inocula were prepared using the procedure described in item 2.1.1 and added to glass vials. The flasks were incubated at 30°C for 28 days and protected from light to avoid photooxidation of the DCPIP [41]. As a positive indication of biodegradability, visual evaluations were performed following the change in color from blue (oxidized) to colorless (reduced) medium, every 24 h until the ninth day.
Microbial growth
To evaluate the growth of the fungi H. resinae (F089) and the isolated yeast, sterile glass vials (150 mL) were prepared containing 100 mL of the aqueous phase (BH medium) [42] and 2 mL of jet fuel, farnesane, and 10% farnesane blend. As a positive control for the experiment, flasks with 102 mL of potato dextrose broth with fungal inoculum were used. The experiment with the fungi was carried out in quintuplicates (for each sampling time) at room temperature 20 ± 2°C. The flasks were kept in the dark during the 28 days of the assay to avoid photooxidation. Fungal inocula were prepared according to item 2.1.1. An aliquot of 2 μL of the aqueous phase from the quintuplicates of each treatment were inoculated in PDA medium (potato dextrose agar) to confirm the viability of the fungi over the evaluation time. At the end of each sampling time, 7, 14, 21, and 28 days of incubation, the aqueous phases (BH medium) [42], and the fuel phase (jet fuel, farnesane, and 10% farnesane blend) were separated using pipettes in sterilized vials (plastic centrifuge tubes and microtubes) respectively. The biomass of H. resinae (F089) and the isolated yeast remained from the separation process of the treatments was recovered by vacuum filtration under asseptic conditions. Filter paper membranes (J Prolab, weight 80, thickness 205 μm, porosity 14 μm) were used to filter the biomass of the fungus H. resinae (F089). The membranes were previously placed in an oven at 30°C for 24 h to remove moisture, and kept in the desiccator for 48 h for later weighing on a precision balance. For the isolated yeast, the same procedure was used, but the membranes were Sartorius brand (25 mm in diameter, 120 μm thick, and porosity of 0.2 μm). The dry weight of the fungal biomass (quintuplicates) was expressed in milligrams and obtained from the difference between the final weight and the initial weight of the membranes, performed on a precision electronic scale (Mars, model AL200C).
Fuel phase
Aliquots of 1mL from the fuel phase of all treatments were collected at 0, 7, 14, 21, and 28 days, and stored at 4°C for further evaluation by gas chromatography. The results presented were the initial time (time zero) and final time for the fungus H. resinae (28 days) and for Exophiala phaeomuriformis (UFRGS Q4.2) (21 days).
Degradation analysis by GC-MS
The degradation profile of fuel hydrocarbons (jet fuel, farnesane, and 10% farnesane blend) by fungi was determined using the gas chromatography technique coupled to a mass spectrometer. The analyses of the fuels in contact with the fungus H. resinae (F089) were performed on an Agilent 7890B high-performance gas chromatograph (HRGC) coupled to a 7000D triple quadrupole mass spectrometer (MS). Helium gas was used as carrier gas with a constant pressure of 60 psiand speed of 29.317 cm/s. The DB-5 20m × 0.1mm × 0.4 μm column was used. The injector temperature was 300°C in split mode, ratio of 200:1, and injection volume of 0.5 μL. The oven initially operated at a temperature of 50°C with a heating rate of 10°C/min to 290°C, totaling 24 min of analysis. The MS was operated in scan mode with m/z range from 17 to 650 u and 70 eV. Chromatographic analysis of the degradation of hydrocarbons by the fungus H. resinae (F089) in the samples of jet fuel, farnesane, and in the 10% farnesane blend was performed at LAMES (Laboratory of Extraction and Separation Methods, Federal University of Goiás, Brazil). The analysis of the degradation profile of hydrocarbons in contact with the isolated yeast was carried out at the LEC (Laboratory of Fuels Tests, Federal University of Minas Gerais). For this purpose, a Shimadzu chromatograph, model GCMS-QP5050, was used. Helium gas was used as carrier gas at a constant flow of 1 mL/min. The Pona 100m × 0.25 mm × 0.5 μm chromatography column was used. The injector temperature was 320°C and the interface temperature was 310°C. Flow splitting of 20 parts (Split 20) was used, with an injection volume of 1.0 μL. In the oven, the following programming was used: isotherm at 60°C for 1 min, followed by a temperature increase to 310°C with a heating rate of 10°C min−1 maintained for 14 min. The analysis time used was 40 min. The MS operated in the m/z range of 45 to 500, from 0.1 to 40 min, with electron impact of 70 eV. Samples of jet fuel, 10% farnesane blend, and farnesane were analyzed at the initial time and after 28 days in contact with the aqueous phase, with and without microorganisms (controls). The results presented are based on the retention times of the majority peaks of the compounds, identified by the NIST libraries. Retention index (RI) for all compounds was determined according to Kovats method using Sigma-Aldrich LRAB9318 n-alkane (C6–C40) standard. The areas of the peaks in percentages were calculated considering the sum of the integrations of each peak as 100%, thus each compound has its relative % area. The mean and standard deviation of each compound from the jet fuel samples, 10% farnesane blend, and farnesane with inocula and controls were compared at the initial and final times to characterize changes in the percentages obtained.
pH analysis
After the separation of the fuel phase, pH measurements of the aqueous phase were performed at sampling times 0, 7, 14, 21, and 28 days for H. resinae and until 21 days for the isolated yeast. Measurements were performed at room temperature with the aid of a digital pH meter (Digimed, model DM-22).
Surface tension measurements
For surface tension measurements, a digital surface tension meter (Gibertini brand, Milan, Italy) was used, adopting the Wilhelmy plate method. Measurements were performed with 10 mL of aqueous phase, after filtration, in the absence of biomass. The samples were left for 30 min at room temperature. To calibrate the device, distilled water (72.0 mN.m−1) was used as liquid standard and ethanol (24.0 mN.m−1). For the fungus H. resinae, the analysis of the surface tension of the aqueous phase was performed at 28 days (final time). For the isolated yeast, the analysis of the surface tension of the aqueous phase was performed at 21 days, as it was the sampling time with the highest biomass produced.
Statistical analysis
The experiments of pH variation, fungal biomass, and surface tension measurements obtained in the different treatments were carried out in quintuplicates. For the gas chromatography tests, triplicates were used. The results obtained in the different treatments were submitted to analysis of variance (ANOVA). Means were compared by Tukey’s test, a 5% significance level. Data were analyzed using the BioEstat 5.0 software program (https://www.analystsoft.com/br/products/biostat/).
Results and discussion
Isolation and identification of Exophiala phaeomuriformis (UFRGS Q4.2)
The yeast E. phaeomuriformis used in this work was isolated from jet fuel and selected for the study due to its ability to grow in fuels (jet fuel, farnesane, and 10% farnesane blend) in a test with redox-DCPIP indicator [43]. Black yeasts comprise a very heterogeneous group of fungi from a taxonomic and phylogenetic point of view, but have in common melanized cell walls and the formation of daughter cells by yeast-like multilateral or polar budding [44, 45]. According to Isola et al. [46], “black fungi” is an umbrella term used to indicate heterogeneous lineages of the subclasses, Chaetothyriomycetidae and Dothideomycetidae. Most black yeasts additionally exhibit mycelial growth [44, 47]. Black yeasts under adverse conditions (high temperatures, ultraviolet radiation, exposure to chemicals, oligotrophic nutrient conditions, or osmotic stress) are capable of producing an extremely tolerant ecotype, expressed as meristematic and strongly melanized muriform cells [48–51]. Matos et al. [52] proposed the nomenclature E. phaeomuriformis, as its most common morphology is yeast-like rather than meristematic. According to Najafzadeh et al. [53], several species of the genus Exophiala are regularly found as agents that cause skin diseases in animals and humans. For Prefaneta-Boldu et al. [45], the ability to utilize hydrocarbons appears to be correlated with virulence in humans, as seen in genetically uncorrelated phyla of hydrocarbonoclatic fungi such as Scedosporium (Microascales) and Exophiala–Cladophialophora (Chaetothyriales).
Assay with the redox indicator DCPIP (2,6 dichlorophenol-indophenol)
The redox indicator 2,6-dichlorophenol indophenol (DCPIP) [41] was added to the mineral medium as a preliminary test of the degradation potential of fuels (jet fuel, farnesane, and 10% farnesane blend) by the fungi H. resinae and E. phaeomuriformis (UFRGS Q4.2), and at different times to observe indicator color change (Table 1). The principle of this technique is based on the microbial oxidation of a carbon source where electrons are transferred to electron acceptors, such as oxygen, nitrate, and sulfate in the respiratory chain. By incorporating an artificial electron acceptor (DCPIP) [41] into the culture medium, it is possible to determine the ability of a microorganism to use a substrate as the color changes from blue (oxidized) to a colorless (reduced) medium due to the flow of electrons from the oxidation of the hydrocarbon by the microorganism [41]. With farnesane, the decolourization time of the indicator by H. resinaewas at day 9, while for the condition with jet fuel and the 10% farnesane blend, the color change was observed in 48 h. Several studies were carried out using redox indicators to evaluate the preliminarydegradation of fuels and oil by microorganisms. Siporin and Cooney [54] were the pioneers in using the redox indicator DCPIP as an indication of the ability of the fungus Cladosporium (H. resinae) to oxidize hexadecane, through the activity of the enzyme succinate dehydrogenase. Miranda et al. [55] used the DCPIP indicator to evaluate yeast strains with the potential to biodegrade diesel oil in samples from the Port of Suape, in Pernambuco, Brazil. The results obtained showed that only 2 of the 23 tested strains were able to discolor the medium in less than 24 h and about 8% discolored the medium in 48 h, a similar result to what happened with the fungus H. resinae (F089), in the jet fuel sample. Similar studies to ours [56, 57] used the DCPIP redox indicator to compare the degradation time in different fuel blends (diesel/biodiesel) using the yeast Candida viswanathii and the bacterium Serratia marcescens, respectively. The results obtained indicated a shorter decolourization time for the indicator (8 h for C. viswanathii and 24 h for S. marcescens). The results of the decolorization of the DCPIP indicator for the yeast E. phaeomuriformis (UFRGS Q4.2) are showed in Table 1. The decolorization time observed was 7 days for all conditions evaluated (jet fuel, farnesane, and 10% farnesane blend).
Table 1.
Preliminary biodegradability test of Hormoconis resinae (F089) and Exophiala phaeomuriformis (UFRGS Q4.2) in the presence of jet fuel, farnesane, and in the 10% farnesane blend with the indicator redox-DCPIP
| Decolourization time (in days) of DCPIP | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatments | 2d | 3d | 4d | 5d | 6d | 7d | 8d | 9d | |
|
Jet fuel Hormoconis resinae |
- | + | + | + | + | + | + | + | + |
| Exophiala phaomuriformis | - | - | - | - | - | - | + | + | + |
|
Farnesane Hormoconis resinae |
- | - | - | - | - | - | - | - | + |
| Exophiala phaeomuriformis | - | - | - | - | - | - | + | + | + |
|
10% Farnesane blend Hormoconis resinae |
- | + | + | + | + | + | + | + | + |
| Exophiala phaeomuriformis | - | - | - | - | - | - | + | + | + |
+Decolourization of the DCPIP indicator during the experiment.
-No decolourization of the DCPIP indicator during the experiment
Fungal growth curve—Hormoconis resinae (F089)
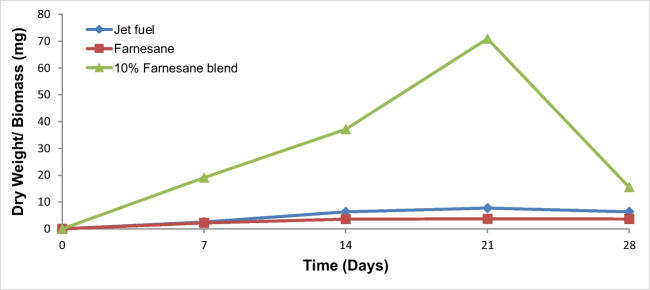
Biomass production of the tratments using jet fuel, farnesane, and 10% farnesane blend with Bushnell and Haas mineral medium as an aqueous phase by the filamentous fungus H.resinae (F089) was measured on days 0, 7, 14, 21, and 28. There was a similar behavior concerning biomass production (increase) in the samples of jet fuel, 10% farnesane blend, and in farnesane over 28 days (Fig. 1). During 7 days, there was an increase in biomass production in jet fuels, and in the 10% farnesane blend, with a statistically significant difference (p<0.05) between the biomass obtained in the farnesane sample (0.3±0.3 mg), and the biomass obtained in the jet fuel samples (4.52±1.0 mg) and the 10% farnesane blend (6.3±1.7). There was no statistically significant difference at 7 days in biomass production between the jet fuel samples and the 10% farnesane blend. At day 14, there was an increase in the production of jet fuel biomass (5.16±3.0 mg) and a decrease in dry weight (2.8±2.0 mg) in the sample of the 10% farnesane blend. There was a statistically significant increase (p<0.05) (from 0.3±0.3to 0.62±0.3 mg) in biomass of farnesane samples from 7 to 14 day. At day 21, an increase in biomass production was observed in the jet fuel samples (8.22±5.0 mg) and 10% farnesane blend (6.5±4.0 mg) with the biomass produced in the farnesane sample (0.62±0.5 mg) similar to day 14. There was a statistically significant difference between the jet fuel and 10% farnesane blend when compared to the farnesane treatment. At the end of 28 days, there was an increase in biomass production in all treatments with a statistical difference among all treatments: jet fuel (19.3±8 mg), 10% farnesane (12.1±3.0 mg), and farnesane (2.2±1.0 mg).
Fig. 1.
Biomass values (mg) from the interface oil- aqueous phase at 0, 7, 14, 21, and 28 days for jet fuel, farnesane, and 10% farnesane blend samples with Hormoconis resinae inoculum
Regarding the biomass production in the sampling times of the jet fuel treatment, significant differences were observed between the test times and the final test time of 28 days (Fig. 2). In the farnesane treatment, low values of biomass were observed. However, a difference was observed related to the production of biomass, with statistical significance between days 7, 14, 21, and day 28. The biomass analyses obtained on days 7, 14, 21, and 28 of the 10% farnesane treatment showed the same trend: there was a statistically significant difference between days 7, 14, 21 compared to day 28.
Fig. 2.
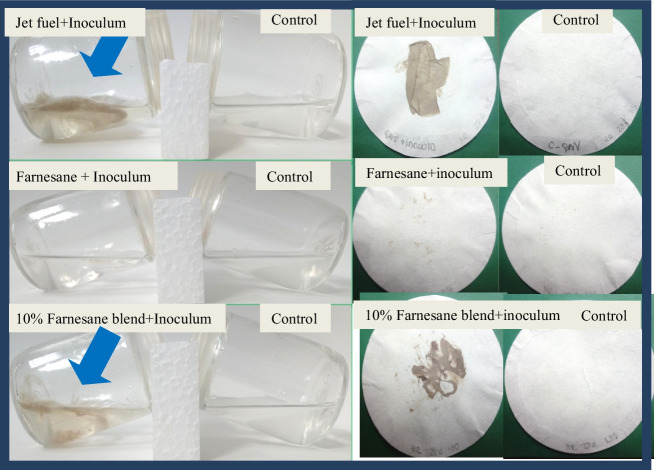
Microcosms’ aspect at 28 days with biomass recovered from interface of jet fuel; farnesane and 10% farnesane blend samples with Hormoconis resinae inoculum. The controls correspond to the sample without inoculation of microorganisms
The ascomycete fungus Amorphotheca resinae (sexual stage) [58] is widely known as H. resinae (asexual stage) or its obligate synonym Cladosporium resinae [24]. H. resinae belongs to Sacchamyceta, Pezizomycotina, Leotiomyceta, Sordariomyceta, Leotiomycetes, Leotiomycetes incertae sedis, and Myxotrichaceae [24]. This species is also known as: “Cresote fungus,” “Kerosene fungus,” or “jet fuel fungus” [59]. H. resinae is recognized as one of the most deteriogenic fungi in fuels, especially in aviation jet fuel, due to its enzymatic competence in metabolizing hydrocarbons (aliphatic, aromatic), resulting in the production of biomass and acidic metabolites [27, 28, 60–64]. Biomass monitoring has been used in several studies to determine the deteriogenic potential of degrading microorganisms in jet fuel samples [27, 63, 65] and in simulated storage of diesel and biodiesel [35, 64, 66–76].
Several studies carried out in the 1970s showed growth (biomass) of the fungus H. resinae in aliphatic hydrocarbon chains above six carbons, such as n-heptane and n-octane [77–79]. Lindley and Heydeman [65] evaluated the use of alkanes (diesel oil) using the fungus Cladosporium resinae (Hormoconis resinae) and observed a longer lag phase, indicating acclimation of the fungus for the use of the substrate. The results showed that only n-alkanes promoted the growth of the fungus H. resinae, with biomass values higher than those found for aromatic compounds (particularly with alkyl side chains). The fungus showed faster growth in octadecane in the solid culture medium and the liquid medium. A lag phase was observed that increased according to the number of carbon chains used as a substrate for the fungus. The authors suggested that the growth rate should only be estimated after acclimation (lag phase) in hydrocarbons.
Cofone et al. [77] evaluated the growth of H. resinae UD42 (jet fuel isolate) in a medium with glucose, a mineral medium with aliphatic hydrocarbons (alkanes, alkenes, branched alkanes), and a mineral medium with aromatic hydrocarbons as being the only carbon sources for 34 days. It was observed that in alkanes C9-C19 (C9–C19), the production of biomass by the fungus H. resinae was considered moderate to high (20–132 mgmL−1). Growth was slower in alkanes than in glucose, but the biomass produced in C9 (68mg), C10 (97mg), C11 (132mg), and C12 (105mg) approached the yield obtained with glucose (C6H12O6: 150 mg). This work also showed that H. resinae grew in “2- methylundecane” (13 mg), but no biomass was detected in using “2,6,10- trimethyldodecane.” Although a low biomass production was observed with isopropyl-benzene (2mg), the presence of methyl branches at the end of the chain (“2,6,11- trimethyl dodecane”) made it difficult for the fungus to metabolize. The authors suggested that the preferential degradation by the fungus occurred in alkanes (saturated) and that was free of ramifications.
In our study, it was also observed that in the farnesane samples, the growth of the fungus H. resinae presented a long acclimation phase of about 21 days (Fig. 1), with the formation of 2.2±1.0 mg of biomass after 28 days. The fungus probably required a longer acclimation phase with the farnesane substrate, which may be associated with the expression of enzymes capable of degrading the hydrocarbon with the ramifications of the compound (farnesane). According to Cofone et al. [77], different isolates of H. resinae may exhibit a lag phase (acclimation phase) when growing on substrates such as hydrocarbons, which can take up to 60 days.
From the first to the second week, a reduction in the biomass value of the fungus H. resinae (6.3 to 2.8 mg) was observed in10% farnesane blend (Fig. 1). This reduction may be the result of some cellular stress related to the lack of expression of branched compound degrading enzymes (farnesane) or it may come from weighing errors. Valle [27] observed in their study which evaluated the growth of filamentous fungi, including H. resinae, that the growth seen in the first 5 days may have been due to the transport of nutrients adhered to the mycelium, which was used as an inoculum, which may have happened in the 10% farnesane blend treatment. Biomass production by the fungus H. resinae was evaluated in media containing nitrate and ammonium for 100 days, with aviation jet fuel as the only carbon source, in microcosms with a capacity of 500mL (60mL jet fuel/ 300 mL aqueous phase). The results obtained from the growth of the fungus H. resinae, in mineral medium–containing nitrate, was above 250 mg of dry weight, while with ammonium sulfate, it was around 150 mg. In our study, the volumes of both the aqueous phase and the fuel phase were different, with a microcosm of 150 mL (2mL of fuel phase/100mL of aqueous phase) as well as with the evaluation time (28 days). In this sense, for these conditions, with the strain of H. resinae a trend towards an increase in biomass was observed in the jet fuel and farnesane treatments during the 28 days (Fig. 1). The only treatment in which there was no continuous increase in biomass was in the 10% farnesane blend, evidenced by the reduction in biomass. The inoculum prepared and used in this work was a suspension of spores, so the lag or acclimation phase was characterized by the time necessary for the germination of these spores to occur, and with the production of hyphae (vegetative part of the fungus) with the expression and production of enzymes necessary for fungal development. In the study carried out by Teh and Lee [79], it was observed that n-hexane exerted an inhibitory effect on spore germination and on the mycelial growth of the fungus H. resinae, and in our study, growth inhibition was not observed in any of the evaluated fuels.
Lindley and Heydeman [80] evaluated the uptake of n-alkanes by the fungus Cladosporium resinae from hydrocarbons, as simple substrates or as mixtures (final total concentration 2%). Turner’s mineral medium was used as the aqueous phase. In addition to alkane absorption assays, studies were also carried out to identify extracellular products. The results showed that the smaller alkanes which removal started first were able to support faster growth or higher biomass yields, so the observed sequence cannot be seen as analogous to classical diauxic growth. The results of absorption tests between alkanes of different lengths, clarify that the initiation of removal of shorter alkanes is competitively favored by absorption mechanisms. A possible explanation for this phenomenon might be based on the chemical nature of larger compounds where molecular fluidity would decrease with increasing carbon chain length, making the passage through lipid membranes physically limiting [24]. In our study, degradation of nonane and decane compounds (medium-sized compounds) was also observed in the assays with jet fuel and 10% farnesane (Tables 3 and 5).
Table 3.
Compounds identified in jet fuel by GC-MS; average peak area percentages at initial time and after 28 days with Hormoconis resinae and negative control (C)
| Compounds | Formula | Jet fuel (initial time) | Jet fuel 28 days | Jet fuel (C) 28 days |
|---|---|---|---|---|
| 1,1,3-Trimethyl-cyclohexane | C9H18 | 1.50±0.06a | 1.45±0.17a | 1.48±0.23a |
| 3-Methyl octane | C9H20 | 1.43±0.02a | 0.9±0.38a | 1.33±0.10a |
| Nonane | C9H20 | 9.33±0.08a | 5.97±2.20b | 9.06±0.42ab |
| 2,6-Dimethyl octane | C10H22 | 1.94±0.07a | 2.0±0.15a | 1.96±0.08a |
| Propylcyclohexane | C9H18 | 3.97±0.12a | 3.85±0.23a | 3.97±0.021a |
| 1-Ethyl-2,3-Dimethylcyclohexane | C10H20 | 3.77±0.15a | 3.14±0.49a | 3.72±0.27a |
| Decane | C10H22 | 10.17±0.02a | 8.45±0.99b | 9.98±0.36a |
| 2,6-Dimethyl nonane | C11H24 | 3.06±0.14a | 3,05±0.57a | 3.26±0.05a |
| 2,6,7-Trimethyl decane | C13H28 | 1.82±0.10a | 1.68±0.23a | 1.92±0.01a |
| Undecane | C11H24 | 10.38±0.24a | 9.83±0.23a | 10.23±0.36a |
| 1-(2-Methylphenyl)-ethanone | C9H10O | 3.84±0.04a | 4.55±0.37b | 4.14±0.05ab |
| Dodecane | C12H26 | 8.87±0.37a | 10.24±1.59a | 8.64±0.41a |
| Tridecane | C13H28 | 5.33±0.08a | 6.82±0.91a | 5.66±0.33a |
| Tetradecane | C14H30 | 2.20±0.02a | 3.22±0.71a | 2.35±0.07a |
*Values with the same superscript (letters: a or b) are statistically indistinguishable from each other by the Tukey test at p > 0.05
Table 5.
Compounds identified in 10% farnesane blend by GC-MS; average peak area percentages at initial time and after 28 days with Hormoconis resinae and negative control (C)
| Compounds | Formula | 10% Farnesane blend (initial time) |
10% Farnesane blend 28 days |
10% Farnesane blend (C) 28 days |
|---|---|---|---|---|
| 1,1,3-Trimethylcyclohexane | C9H18 | 1,39±0.05a | 1,06±0.13b | 1,10±0.08b |
| Nonane | C9H20 | 7,70±0.04a | 5,98±0.08b | 6,80±0.37c |
| 2,6-Dimethyl octane | C10H20 | 1,66±0.01a | 1,51±0.09a | 1,55±0.08a |
| Propylcyclohexane | C9H18 | 3,44±0.05a | 3,08±0.16a | 3,15±0.21a |
| 1-Ethyl-2,3-Dimethylcycloxexane | C10H20 | 3,07±0.08a | 3,02±0.13a | 2,09±0.18a |
| Decane | C10H22 | 7.65±0,01a | 7.08±0,05b | 7.31±0,08c |
| 2,6,7-Trimethyldecane | C13H28 | 1,57±0.04a | 1,82±0.04b | 1,66±0.03c |
| Undecane | C11H24 | 7,41±0.08a | 7,43±0.08a | 7,38±0.09a |
| 1-(2-Methylphenyl)-Ethanone | C9H10O | 2,68±0.04a | 3,19±0.09b | 3,03±0.12b |
| Dodecane | C12H26 | 6,53±0.02a | 6,88±0.22a | 6,75±0.13a |
| Tridecane | C13H28 | 3.87±0,05a | 4.15±0,11b | 4.07±0,12ab |
| 2,6,10-Trimethyldodecane | C15H32 | 22,37±0.08a | 22,88±0.35a | 22,98±0.55a |
*Values with the same superscript (letters: a or b) are statistically indistinguishable from each other by the Tukey test at p > 0.05
Fungal growth curve—Exophiala phaeomuriformis (UFRGS Q4.2)
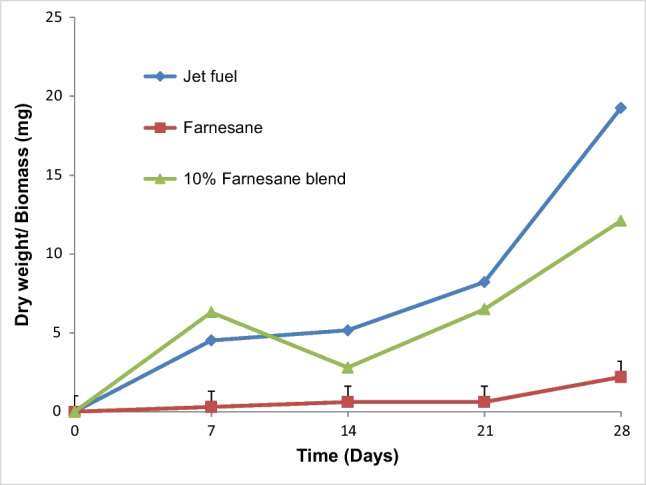
The biomass values produced over the 28 days are shown in Fig. 3. At day 7, the biomass produced by the yeast fungus E. phaeomuriformis (UFRGS Q4.2) in the 10% farnesane blend and farnesane samples was 2.5 (±0.98) and 2.2 (±1.2) milligrams respectively, while in the sample of the 10% farnesane blend it was about 8 times higher (19.1± 6.9 mg). At day 14, a 2.5-fold increase in the biomass of the yeast fungus E. phaeomuriformis (UFRGS Q4.2) was observed in the jet fuel treatment (6.3±0.5 mg) when compared to the time of 7 days (2.5 ±0.98 mg). The biomass formed under the test conditions with 10% jet fuel blend at 14 days was about 2 times higher (39.1 ± 7.1 mg) than the biomass obtained at 7 days (19.1 ± 6.9 mg), and the biomass formed in the farnesane treatment was about 1.5 times higher (3.6 ± 1.7 mg) compared to the production at 7 days (2.2 mg). At day 21, the biomass formed in the 10% farnesane blend was (70.9± 26.4 mg), the highest observed during the growth curve and was about 2 times higher than at day 14 (39.1 mg). The mean biomass obtained in the jet fuel samples was 7.8 ± 1.1 mg, while the mean biomass obtained in the farnesane treatment samples was 3.8 ± 2.4 mg. On the twenty-eighth day (final time of the study), a reduction in the mean biomass of jet fuel treatments (from 7.8 to 6.3 ± 1.6) and 10% farnesane blend (from 70.9 to 15.5 ± 2.1) was observed, while in the farnesane treatment the average biomass remained similar (3.7± 0.8 mg).
Fig. 3.
Biomass values (mg) from the interface oil- aqueous phase at 0, 7, 14, 21, and 28 days for jet fuel, farnesane, and 10% farnesane blend samples with Exophiala phaeomuriformis inoculum
In the evaluation of the biomass averages between the sampling times in the jet fuel treatments, farnesane, and 10% farnesane blend, it was observed that there was a difference with statistical significance (p<0.05) between day 7 and the other sampling times (14, 21, and 28, respectively) for jet fuel treatment. No statistically significant difference was observed between the sampling times of the farnesane treatment. Regarding the average biomass of the sampling times of the 10% farnesane treatment, differences with statistical significance were observed between days 7, 14, and 28 when compared to the average biomass obtained on day 21. Statistically significant differences were observed (p< 0.05) between the biomass values produced in jet fuel and farnesane in relation to the 10% farnesane treatment by Tukey’s test at all test times (7, 14, 21, and 28 days). However, no statistically significant differences (p<0.05) were observed in biomass production between jet fuel and farnesane treatments. In this sense, the twenty-first day of incubation was chosen among all treatments to evaluate changes caused by growth in the aqueous phase as it was the sampling time showing the highest biomass recorded. Analyses such as pH and surface tension were chosen for the analysis of degradation of hydrocarbons. Figure 4 shows the appearance of microcosms (on the left) and biomass recovered in filter membranes at the interface (on the right) with jet fuel; farnesane and the 10% farnesane blend in BH mineral medium with the yeast fungus E. phaeomuriformis (UFRGS Q4.2) after 21 days. In Fig. 4, the interface region is indicated with an arrow, where the greatest growth (biomass) can be observed in the 10% farnesane blend.
Fig. 4.
Microcosms’ aspect at 28 days with biomass recovered from the interface in jet fuel; farnesane and 10% farnesane blend samples with Exophiala phaeomuriformis. The controls correspond to the sample without inoculation of microorganisms
pH analysis of the aqueous phase
pH measurements of the aqueous phase from assays with H. resinae (F089) and for the yeast E. phaeomuriformis (UFRGS Q4.2) were performed at sampling times 0, 7, 14, 21, and 28 days (Table 2). The pH measurements of the aqueous phase of the treatments jet fuel, farnesane, and 10% farnesane with and without the inoculum of the fungus H. resinae (controls) showed a statistical difference (p>0.05) when compared to the initial pH value (7.2) of the mineral medium Bushnell and Haas [42]. There was a significant decrease in pH (p>0.05) in the aqueous phase of the treatments jet fuel and 10% farnesane blend when compared to their respective negative controls (without inoculum) as shown in Table 2. There was no statistically significant difference between farnesane treatment and control. Thus, the main decrease observed was in the condition with jet fuel and the fungus H. resinae (6.5 to 4.3). In the 10% farnesane blend with the fungus, the decrease was from 6.6 to 5.9. These results may be related to an increase in the concentration of acidic metabolites since fungal growth was confirmed by the increase in biomass in the treatments mentioned above, 19.3 mg in the jet fuel and 12.1 mg in the 10% farnesane treatment as can be seen in Fig. 2. Cooney and Prooby [78] evaluated the fungus H. resinae growing under different types of isolated hydrocarbons and in aviation kerosene (pure Jet-A) with Bushnell and Haas mineral medium as the aqueous phase. Their study showed a largest pH decrease of 4.2 in the Jet-A condition.
Table 2.
Values of surface tension and pH measurements of the aqueous phase of jet fuel, farnesane, and 10% farnesane blend samples after 21 days (Exophiala phaeomuriformis) and 28 days (Hormoconis resinae) under incubation. The control samples were microcosms without microrganisms
| Treatments Bushnell–Haas (T0) | Surface tension *BH medium T0: 57.4 mN. m−1 | pH* BH medium T0: 7.2 | ||
|---|---|---|---|---|
| Jet fuel | ||||
| Hormoconis resinae | 48.8 | ±3.5a | 4.30 | ±0.35a |
| Exophiala phaeomuriformis | 53.3 | ±5.6a | 6.88 | ±0.15d |
| Jet fuel control | ||||
| Hormoconis resinae | 44.1 | ±2.3b | 6.50 | ±0.28b |
| Exophiala phaeomuriformis | 51.5 | ±9.9 a | 7.00 | ±0d |
| Farnesane | ||||
| Hormoconis resinae | 50.9 | ±4.4c | 6.65 | ±0.02c |
| Exophiala phaeomuriformis | 45.4 | ±4.4b | 6.97 | ±0e |
| Farnesane control | ||||
| Hormoconis resinae | 45.5 | ±3.5c | 6.69 | ±0.05c |
| Exophiala phaeomuriformis | 43.2 | ±3.5 b | 6.93 | ±0e |
| 10% Farnesane blend | ||||
| Hormoconis resinae | 46.4 | ±1.4d | 5.95 | ±0.20d |
| Exophiala phaeomuriformis | 45.9 | ±10 c | 6.98 | ±0.12f |
| 10% Farnesane blend control | ||||
| Hormoconis resinae | 44.6 | ±3.0d | 6.51 | ±0.19e |
| Exophiala phaeomuriformis | 44.5 | ±0.4 c | 6.94 | ±0f |
*Values with the same superscript (letters: a, b, c, d, f) are statistically indistinguishable from each other by the Tukey test at p > 0.05
Cofone et al. [77] evaluated the growth of H. resinae in different hydrocarbons (alkanes, branched alkanes, aromatics, and polycyclic aromatics), and observed that the biomass yield at the end of 34 days was dependent on the hydrocarbons and cultivation conditions. No growth of H. resinae was observed in “2,6,10-trimethyl dodecane” evaluated in this study. It was observed that the lowest pH values were recorded in static cultures than in agitation, indicating that the culture conditions can also affect the production of acidic metabolites. The highest pH decrease rates were observed for growth on undecane (6.0 to 2.5) decane (6.0–2.7) and dodecane (6.0–3.0). The authors did not assess spore viability under conditions in which there was no growth [77]. Our results showed the growth of H. resinae in n-alkanes and a decrease of pH in the jet fuel and 10% farnesane treatments. Likewise, with “2,6,11-trimethyl dodecane,” it was not possible to obtain biomass values even close to those observed for kerosene (alkanes).
According to Martin-Sanchez et al. [62], the action of organic acid metabolites produced by the fungus H. resinae can cause microorganism-induced corrosion (MIC) in storage and distribution systems. For Krohn et al. [6], organic acids produced by fungi correspond significantly to corrosion influenced by microorganisms. The biocorrosion of aluminum and its alloys has been attributed to microbial contamination caused by the fungi Amorphoteca resinae (Hormoconis), Aspergillus fumigatus, and Penicillium corylophilum [6, 79].
Regarding the yeast E. phaeomuriformis (UFRGS Q4.2), there was no significant decrease (p>0.05) in the measurements of pH of the aqueous phase in the treatments jet fuel, farnesane, and 10% farnesane blend when compared to their respective controls by the Tukey test, at all times.
In a study about the susceptibility of different alternative aviation fuels to microbial growth was evaluated, Hill [33] used purified water without inorganic salts as an aqueous phase, where there was no pH buffering. According to the author, purified water can be expected to be weakly acidic (due to the absorption of CO2 from the air). The results obtained showed that the pH of the aqueous phase in all microcosms remained at 5.5 for the first 28 days, exactly the same time as in our study. Comparing these results with ours, the BH mineral medium used to provide balance of nutrients (such as phosphates—KH2PO2 and K2HPO4) to the microbial cell is also a buffering system [81]). The study by Hill [33] provided an important piece of data related to the pH variation of the aqueous phase. The pH of the aqueous solution at day 49 in Jet-A-1 microcosms treated with MEROX (a treatment used to reduce corrosive compounds in aviation fuel) dropped to 3.5 while the pH in other microcosms remained at 5.5 throughout the study (even without buffering).
Another possible explanation (in addition to buffering the BH medium) for not having observed significant decreases in pH in the aqueous phase of the conditions evaluated in this study is due to the physiology of the black yeasts, such as E. phaeomuriformis (UFRGS Q4.2). According to Sterflinger [47], an energy and nutrient demand is necessary for the synthesis of melanins, trehalose, and phenols necessary for survival in predominantly oligotrophic habitats, which do not allow organic acids and/or secondary metabolites or any metabolic product to be produced or excreted.
Surface tension measurements of the aqueous phase
The surface tension measurements of the aqueous phase for the fungus H. resinae were performed at 28 days, and for the yeast E. phaeomuriformis (UFRGS Q4.2) at 21 days (Table 2). A reduction in the surface tension of the aqueous phase (p>0.05) was observed in the presence of jet fuel, farnesane, and 10% farnesane with inoculum of the fungus H. resinae as well as in the controls when comparing the surface tension of the BH medium (57.4). Regarding the surface tension of the aqueous phases of the treatments with the inoculum of the fungus H. resinae, there was no statistically significant reduction (p>0.05) in the samples with the farnesane fuels and 10% farnesane mixture when compared to controls (without inoculum). It was only in the jet fuel and control treatments where there was greater biomass growth that a reduction in surface tension was observed, with statistical significance (p>0.05). At this sampling time, the reduction of nonane and decane compounds by GC-MS was also determined, suggesting a possible increase in the bioavailability of these hydrocarbons, due to the production of biosurfactant by the fungus. Biosurfactants can increase the solubilization and dispersion of hydrocarbons, by reducing the surface tension, which can increase the affinity between a microbial cell and an organic compound [82–84]. Regarding the yeast fungus E. phaeomuriformis (UFRGS Q4.2), there was no significant decrease (p>0.05) in surface tension measurements between treatments jet fuel, farnesane, and 10% farnesane blend and controls by Tukey’s test. However, a statistically significant variation (p>0.05) was observed between the aqueous phases of the farnesane treatments (inoculum and control) when compared to the initial surface tension of the BH medium (57.4).
Studies conducted by Cooney and Prooby [78] with H. resinae observed a low concentration of surfactants produced by the fungus, being characterized as fatty acids and phospholipids. Lindley and Hendeyman [80] in a study on the metabolism of the fungus H. resinae in mixed alkanes, supplementing with phospholipids (similar to those produced during growth in alkanes) observed an increase in the removal rate of the evaluated hydrocarbons. The study showed that the uptake of dodecane, hexadecane, and octadecane from a mixture can be by a common mechanism, the order being determined, by competition, by the affinity of the system for each alkane. Muriel et al. [85] evaluated the growth of H. resinae in Bushnell–Haas [42] and JP-8 medium as the only carbon source for 25 days and recorded a reduction from 72 to 50 mN/m, with confirmation of the production of an extracellular surfactant characterized as cladosan.
Reductions in surface tension measurements were observed in the control (without fungi). Jet fuel is also known for its action as an industrial solvent in cleaning the most diverse products, but it is considered insoluble in water. According to Cazarolli et al. [75], the reduction in surface tension measurements of the mineral medium in contact with biofuels observed in a study, in the control treatments, is due to the nature of the molecules that make up the biofuel, as in the case of biodiesel from soybean, with surfactant action.
Regarding the yeast E. phaeomuriformis (UFRGS Q4.2), it showed no significant decrease (p>0.05) in the surface tension measurements among treatments jet fuel, farnesane, and 10% farnesane blend and their respective controls by the test of Tukey.
Degradation of hydrocarbons by GC-MS
Chromatographic analyses of biodeterioration studies of fuels have been used as important analytical support as it allows us to know the profile of hydrocarbons present in fuelsin the presence and absence of deteriogenic microorganisms. Therefore, it is possible to know about the preferred degradation of some hydrocarbons by microorganisms in the complex mixture such as fuels [17, 32, 76, 86–90].
Hormoconis resinae (F089)
Jet fuel composition analysis where the major compounds were evaluated using gas chromatography showed the presence of aromatic compounds, normal alkanes, cycloparaffins, and isoparaffins (Table 3). The results of degradation, obtained by GC-MS, of the hydrocarbons presents in the condition with pure jet fuel with the fungus H. resinae after 28 days, indicated a reduction mainly in the area of the decane compound (Table 3 and Fig. S1). The reductions observed for the hydrocarbons were calculated always in relation to the observed in the control and calculated if the difference was significantly accepted. No reduction in the fraction of aromatic hydrocarbons was observed. Table 5 shows the evaluated hydrocarbons, and no significant reductions were observed in their concentrations when compared to the control.
In the pure farnesane condition, chromatographic analysis (GC-MS) did not show degradation of the compound “2,6,10- trimethyl dodecane” (Table 4 and Fig. S2). However, in some replicates, a greater reduction in area was observed in the control compound (without inoculum) than in the treatment with inoculum, which may be attributed to a problem during the analytical procedure. Another possibility can be attributed to the process of degradation of farnesane in contact with the mineral medium, but the reactions should have also occurred in the treatment where the fungus was inoculated. In this way, we conclude that farnesane was not used by the fungus as a carbon source, but also it was not toxicto the spores, since the spores remained viable during the two 28 days of simulated storage.
Table 4.
Compounds identified in farnesane by GC-MS; average peak area percentages at initial time and after 28 days with Hormoconis resinae and negative control (C)
| Compounds | Formula | Farnesane (initial time) |
Farnesane 28 days |
Farnesane (C) 28 days |
|---|---|---|---|---|
| 2,3,7-Trimethyloctane | C11H24 | 0.24±0.01a | 0.25±0.01a | 0.30±0b |
| 2,6,10-Trimethyldodecane | C15H32 | 98.01±0.02a | 97.93±0.01b | 97.79±0c |
| 1-(1,5-dimethylhexyl)-4-Methylbenzene | C15H24 | 0.99±0.01a | 1.00±0.01a | 1.1±0b |
*Values with the same superscript (letters: a, b, or c) are statistically indistinguishable from each other by the Tukey test at p > 0.05
Analysis of the composition of the 10% farnesane blend showed the presence of normal alkanes, cycloparaffins, isoparaffins, and aromatic and farnesane compounds. The results of the monitored compounds of the mixture are shown in Table 5 and Fig. S3. A reduction in the peak area of the compound “1,1,3-trimethyl cyclohexane” (inoculum and control) was observed which suggested abiotic degradation. The nonane and decane compounds had a statistically significant reduction in area when compared to the control which suggesteddegradation by the fungus (biotic).
According to Smart et al. [88], since diesel and jet fuel are middle distillates and can be a carbon source for microorganisms, it is possible that the rapid growth of these populations lead to variations in the composition of fuels. Microorganisms can produce residues at levels from ppb to ppm that can be important to the overall properties of the fuel. However, it is critical to understand whether contamination of 1 ppm can lead to failure in specification tests for properties, such as thermal stability or the presence of gum, as measured by ASTM D381. Smart et al. [88] proposed in the experimental design of studies with jet fuel and diesel, which involve the support of analytical techniques (GC, GC-MS, and GC-GC), special attention to the proportion of the aqueous/fuel phase used. Small variations produced by microorganisms may be indistinguishable if the volume of fuel is much greater than that of the aqueous phase. Therefore, it is suggested that the ratios are 1:10 fuel/aqueous phase or less than 1:50 or 1:100. Higher ratios such as 1:5 or 1:1 make it more difficult to prove and possibly visualize reactions resulting from bacterial metabolism.
In our study, proportions of 1:50 were used, with 2 mL of fuels (jet fuel, fernesane, and 10% farnesane blend) for 100 mL of aqueous phase, a proportion suggested by the study by Smart et al. [88]. However, in a study carried out in 2012, Hill [33] used fuel/water ratios of 1000:1. The explanation for this correlation is based on the fact that if the volume of fuel in relation to water is not high, the water-soluble components will migrate from the fuel to the aqueous phase. Therefore, the concentrations of the compounds in the aqueous phase will not reflect those found in real fuel tanks.
Interest in the degradation of aliphatic compounds by fungi increased sharply from the 1960s onwards with the advent of jet aircraft and, consequently, the shift from gasoline to kerosene-based fuels [45]. Hu et al. [3] suggested the existence of a symbiotic metabolism, in which fungi break down less soluble compounds into more soluble, which can then be consumed by bacteria. Shapiro et al. [29] analyzed isolated fungi of two different types of aviation fuel and detected the presence of amplicons of the rRNA gene 18 S and 16S rRNA in hyphae. This result associated the presence of bacteria with mycelium fungus, in a clear action of cometabolism. According to the authors, the mycelium fungi of the genera Talaromyces, Penicillium, and Aspergillus would serve as a transport vector for the dispersal movement of bacteria by “fungal highways” and for the transport of hydrocarbons and metabolites by “fungal pipelines” providing a favorable microenvironment for bacteria in its hyphosphere. That characteristic of transport would be essential for an establishment of a constanted metabolic crosstalk. The conclusion obtained according to the authors is that only isolates of micromycetes existing in communities with bacteria are effective in terms of degradation of TS-1 jet fuel.
Most fungi grow at the oil-water interface that accumulate inside fuel storage tanks as a result of water condensation and use hydrocarbons as a source of carbon and energy. Microbial activity and growth occur in the presence of water, and only microorganisms with sporulated (dormant phase) forms are possible in the fuel phase. Chemical contaminants and water can provide the nitrogen, phosphorus, sulfur, and other micronutrients (trace elements) necessary for biological growth [5, 8, 9, 33, 61, 64, 88]. According to the literature, H. resinae preferentially degrades n-alkanes [27, 64, 65, 80].
Oh et al. [91] conducted a study using the Bushnell–Haas medium as an aqueous phase with 1% (v/v) hydrocarbons (C8–C16) and aromatic hydrocarbons. The results obtained showed low rates of degradation of hydrocarbons, by the strain of H. resinae (NK1), such as decane and undecane when compared to the compounds dodecane, tetradecane, pentadecane, and hexadecane. No degradation of aromatic hydrocarbons was observed. Maciel et al. [92] also used the coupled gas chromatography technique and mass spectrometry to evaluate the kinetics of aviation jet fuel degradation by the fungus Penicillium spp. during a soil bioremediation trial. The results showed the degradation of alkanes such as nonane, decane, undecane, dodecane, tridecane, tetradecane, and pentadecane. Khan et al. [93] evaluated the degradation of kerosene by the fungus Penicillium janthinellum, after 16 days, with the coupled gas chromatography technique and mass spectrometry, and the biodegradation was confirmed with the removal of hydrocarbons in the range of C8–C18. In our study, we highlight the compound dodecane, used by the fungus H. resinae, as within the preferential degradation range of kerosene by other filamentous fungi, according to the referenced literature.
Depending on the isolate, that is, on the variety of H. resinae, different periods can be observed regarding the acclimation phase to the type of hydrocarbon (degree of saturation, solubility; position and number of ramifications) that is being used as a carbon source and the conditions of cultivation (static, aerated; proportions of fuel phase and aqueous phase; support of macro and micronutrients) [8, 17, 27, 61, 64, 65, 69, 77, 80, 86–88].
According to Rafin and Veigni [24], after the passive adsorption of n-alkanes on the cell membrane, their entry into the cytoplasm seems to require energy consumption. Although the exact mechanism by which the n-alkanes are transported to the fungal cells is not completely elucidated. Lindley [94] suggests that these compounds are apparently assimilated via active transport.
Kronh et al. [6] carried out studies of transcriptomic analysis of the genome of the fungus H. resinae that showed the mapping of more than 15,441 RNA readings related to the enzymes, aldehyde dehydrogenase and cytochrome P450. These enzymes are potentially involved in oxidative processes that may be linked to kerosene degradation. Previous studies [95, 96] had already pointed out that the initial “attack” on n-alkanes usually started from the terminal methyl group by a constitutive n-alkane monooxygenase [24]. This enzyme is NADH-dependent and belongs to the P450 class of monooxygenases [24, 95, 96]. Subsequent catabolic steps utilize enzymes specific for long-chain alcohols (fatty alcohol oxidase) and aldehyde dehydrogenase) to produce fatty acid. Goswami and Cooney [96] located fatty aldehyde dehydrogenase in the fungal cytoplasm and fatty alcohol oxidase bound to the mitochondrial membrane [24].
Exophiala phaeomuriformis (UFRGS Q 4.2)
The results obtained by GC-MS of degradation of thejet fuel hydrocarbons with the fungus E. phaeomuriformis after 21 days (Table 6 and Fig. S4) indicated the reduction of the peak areas of the compounds: methyl cyclohexane, “2,5-dimethyl hexane,” “1,3-dimethyl cyclohexane,” octane, “2,6-dimethylheptanes,” “1,1,3-trimethylcyclohexane,” “2,5-dimethyl heptanes,” nonane, “2,6-dimethyl octane and “2-methyl nonane,” but with no statistical difference with the control, indicating abiotic degradation. Increases were observed in the areas, with statistical significance of the compounds: “4-methyl decane,” undecane, dodecane, tridecane, tetradecane, and pentacosan in the control and inoculum treatments.
Table 6.
Compounds identified in jet fuel by GC-MS; average peak area percentages in the initial time and after 21 days with Exophiala phaeomuriformis and negative control (C)
| Compounds | Formula | Jet fuel (initial time) | Jet fuel 28 days | Jet fuel (C) 28 days |
|---|---|---|---|---|
| Cyclohexane | C6H12 | 0.21±0.01a | 0.09±0.07a | 0.12±0.09a |
| Methyl cyclohexane | C7H14 | 0.21±0.02a | 0.01±0.00b | 0.02±0.01b |
| 2,5-Dimethyl hexane | C8H18 | 0.32±0.02a | 0.05±0.01b | 0.06±0.03b |
| 1,3-Dimethyl cyclohexane | C8H14 | 0.45±0.03a | 0.08±0.02b | 0.08±0.04b |
| Octane | C8H18 | 1.16±0.03a | 0.32±0.07b | 0.39±0.14b |
| 2,6-Dimethyl heptane | C9H20 | 0.62±0.02a | 0.28±0.05b | 0.32±0.07b |
| 1,1,3-Trimethyl cyclohexane | C9H18 | 2.15±0.11a | 0.93±0.17b | 1.00±0.30b |
| 2,5-Dimethyl heptane | C9H20 | 4.17±0.19a | 2.07±0.27b | 2.24±0.48b |
| Nonane | C9H20 | 17.70±0.11a | 10.95.±0.82b | 11.55±1.19b |
| 2,6-Dimethyl octane | C10H22 | 3.93±0.08a | 3.07±0.25b | 3.31±0.18b |
| 2-Methyl nonane | C10H22 | 4.72±0.09a | 4.17±0.21b | 4.22±0.21b |
| Decane | C10H22 | 23.58±0.38a | 22.94.±0.17a | 22.99±0.67a |
| 4-Methyl decane | C11H24 | 3.73±0.23a | 4.71±0.36b | 4.35±0.15ab |
| Undecane | C11H24 | 16.26±0.51a | 20.05.±0.64b | 18.60±0.70b |
| Dodecane | C12H26 | 13.12±0.55a | 16.45.±2.92ab | 17.91±0.36b |
| Tridecane | C13H28 | 4.42±0.06a | 7.53±0.38ab | 7.08±0.70b |
| Tetradecane | C14H30 | 2.48±0.04a | 4.85±0.45ab | 4.48±0.51b |
| Pentacosane | C25H52 | 0.75±0.01a | 1.45±0.13ab | 1.29±0.20b[ |
*Values with the same superscript (letters: a or b) are statistically indistinguishable from each other by the Tukey test at p > 0.05
Regarding the compound “2,6,10-trimethyldodecane” (Table 7 and Fig. S5), no reductions were observed in the chromatogram areas of the conditions with the yeast E phaeomuriformis nor the respective control.
Table 7.
Compounds identified in farnesane by GC-MS; average peak area percentages at initial time and after 21 days with Exophiala phaeomuriformis and negative control (C)
| Compounds | Formula | Farnesane (Initial time) | Farnesane 28 days | Farnesane (C) 28 days |
|---|---|---|---|---|
| 2,6,10-Trimethyldodecane | C15H32 | 100,00a | 100,00a | 100,00a |
*Values with the same superscript (letter: a) are statistically indistinguishable from each other by the Tukey test at p > 0.05
In the 10% farnesane blend samples (Table 8 and Fig. S6), a reduction was observed in the areas of the compounds: methyl cyclohexane, “2,5-dimethyl hexane,” “1,3-dimethyl cyclohexane,” octane, “2,6-dimethyl heptanes,” and “1,1,3-trimethyl cyclohexane” but no statistical difference related to the inoculum and control treatments. In the 21 days of treatment (with inoculum) and control, an increase was observed in the areas of the compounds: undecane, dodecane, tridecane, “2,6,10-trimethyl dodecane,” tetradecane, and pentacosan.
Table 8.
Compounds identified in 10% farnesane blend by GC-MS; average peak area percentages at initial time and after 21 days with Exophiala phaeomuriformis and negative control (C)
| Compounds | Formula | 10% Farnesane blend (initial time) | 10% Farnesane blend 28 days |
10% Farnesane blend (C) 28 days |
|---|---|---|---|---|
| Cyclohexane | C6H12 | 0.17±0.01a | 0.08±0.07a | 0.07±0.05a |
| Methyl- cyclohexane | C7H14 | 0.10±0.01a | 0.02±0.01b | 0.03±0.02b |
| 2,5-Dimethyl hexane | C8H18 | 0.15±0.01a | 0.05±0.03b | 0.06±0.03b |
| 1,3-Dimethyl ciclohexane | C8H14 | 0.21±0.01a | 0.09±0.04b | 0.09±0.04b |
| Octane | C8H18 | 0.52±0.01a | 0.31±0.11b | 0.31±0.07b |
| 2,6-Dimethyl heptane | C9H20 | 0.29±0.01a | 0.23±0.05ab | 0.22±0.03b |
| 1,1,3-Trimethyl ciclohexane | C9H18 | 0.99±0.02a | 0.75±0.18ab | 0.70±0.09b |
| 2,5-Dimethyl heptane | C9H20 | 1.91±0.11a | 1.70±0.30a | 1.56±0.21a |
| Nonane | C9H20 | 8.23±0.06a | 8.26.±0.90a | 7.51±1.17a |
| 2,6-Dimethyl octane | C10H22 | 2.02±0.01a | 2.1±0.25a | 2.03±0.27a |
| 2-Methyl nonane | C10H22 | 2.41±0.13a | 2.85±0.16a | 2.60±0.34a |
| Decane | C10H22 | 13.28±0.13a | 15.11.±1.69a | 16.04±0.52a |
| 4-Methyl decane | C11H24 | 2.28±0.10a | 3.02±0.21b | 2.40±0.31a |
| Undecane | C11H24 | 9.70±0.64a | 11.85.±0.67b | 11.78±0.44b |
| Dodecane | C12H26 | 9.10±0.30a | 12.05.±0.63b | 12.33±1.43b |
| Tridecane | C13H28 | 3.50±0.08a | 4.82±0.18b | 5.04±0.32b |
| 2,6,10-Trimethyl Dodecane | C15H32 | 42.9±0.67a | 32.38±1.36b | 34.73±3.46b |
| Tetradecane | C14H30 | 1.60±0.02a | 2.46±0.21b | 2.53±0.14b |
| Pentacosane | C25H52 | 0.61±0.04a | 0.97±0.10b | 0.91±0.12b |
*Values with the same superscript (letters: a or b) are statistically indistinguishable from each other by the Tukey test at p > 0.05
Hashen et al. [97] evaluated the biodegradation and detoxification of aliphatic and aromatic hydrocarbons by yeasts in microcosms with minimal mineral medium (aqueous phase) and octane as a carbon source. The results obtained showed that octane promoted continuous growth of all yeasts (OD 600 nm) and that more specifically, the yeast Rhodotorula taiwanensis KKUY-0162 can efficiently utilize octane, breaking it down into many transient compounds and rebuilding it into various metabolites. secondary. According to Maier [98], the chemical structure of the organic compound is crucial for biodegradation. The degradation of hydrocarbons by microorganisms can be hampered by the presence of branched and functional groups. When the reaction site is blocked by a functional group or a branch, contact between the contaminant and the enzyme at the reaction site is prevented. Functional or branched groups can also affect substrate transport across the cell membrane, especially if the transport is aided by an enzyme. For Sutton et al. [90], branching makes both initial oxidation and subsequent lipid catabolism difficult, apparently because tertiary and quaternary carbon atoms interfere with steric activity. The first indication of the biodegradation of fuels typically involves the selective removal of C6–C17 alkanes and small aromatics. As biodegradation continues saturated hydrocarbons outside the initial range (C6–C17) are selectively removed. Normal alkanes are removed more quickly than mono and millimethyl alkanes. In this sense, a wide variety of alternative fuels produced from many raw materials, processes, and conditions may contain minimal amounts of n-alkanes and high levels of branched alkanes, products of the specific processing conditions used to generate these fuels [99].
Farnesane “2,6,10-trimethyl dodecane” is a so-called “drop-in” biofuel that can be mixed with conventional aviation kerosene up to a specific proportion, allowing the same existing supply infrastructure to be used [100]. In our studies, we used biokerosene (farnesane) pure and mixed in a 10% fraction with aviation kerosene.
Stamper et al. [89] when analyzing by GC-MS different types of diesel and biodiesel with marine microbiota inoculum (for 25 days) indicated that chemical analyzes did not show changes for various fuel classes in relation to negative controls, such as such as n-alkanes, isoalkanes, alkyl monocycloalkanes, esters, and branched alkanes. Branched alkanes, due to the steric hindrance caused by branching, are considered less readily biodegradable than n-alkanes, with highly branched alkanes being more recalcitrant to biodegradation. Still regarding the degradation of biofuels, Striebich et al. [99] evaluated the growth of Pseudomonas aeruginosa ATCC 33988 in aviation kerosene (Jet-A) and alternative fuels SPK and Isopar-M. The authors observed the preferential consumption of n-alkanes, followed by cyclic alkanes, and the degradation of branched (isoaparaffins) and aromatic hydrocarbons was not observed.
In our study, we compared the growth of the fungus H. resinae in farnesane treatment (2 mg of dry weight) with that recorded in jet fuel (19.3 ± 8), we found low biomass production after 28 days. Probably the fungus does not have genes or there was no gene expression related to enzymes capable of metabolizing the branched hydrocarbon fraction. However, in the study by Striebich et al. [99], when 5% (v/v) hexadecane was added to SPK fuels and Isopar-M, Pseudomonas bacteria grew until the hexadecane was completely consumed. The addition of hexadecane to isoparaffinic fuels also revealed that the lack of growth in SPK or Isopar-M was not caused by any toxicity of the biofuels, but by the lack of enzymatic competence in using them as a carbon source. These results can also be compared to our study, as we verified that the mixture with 10% farnesane blend promoted the production of biomass, higher (12.1±3.0 mg) than in farnesane condition. These evidences suggest that the branched hydrocarbons did not have a toxic effect, but they cannot be considered a preferentially degradable carbon source for these strains, due to the lack of enzymatic competence. In our study, the spores remained viable in jet fuel, farnesane and in the 10% farnesane blend during 28 days, as demonstrated by the results obtained from inoculation and detected growth of fungi in appropriate culture medium (potato dextrose agar). Isoparaffin fuels are an important class within a range of potential alternative fuels, but they are not the only alternative fuels being considered for use in aircraft.
Conclusion
The growth of the fungi E. phaeomuriformis (UFRGS Q 4.2) and H. resinae (F089) was evaluated in jet fuel, farnesane, and the mixture 10% farnesane blend for 21 and 28 days, respectively. The highest biomass formation was observed in jet fuel for H. resinae (F089) 19 mg mL−1 and in 10% farnesane blend for E. phaeomuriformis (UFRGS Q 4.2) 70 mg mL−1. The greatest decrease in pH (7.2–4.8) was observed after 28 days in the microcosm with jet fuel and H. resinae (F089). No significant decreases in pH and surface tension were observed for E. phaeomuriformis (UFRGS Q 4.2) under all conditions evaluated with the fuels. H. resinae preferentially degraded C9–C12; however, no degradation of the evaluated hydrocarbons was detected in the experiment using E. phaeomuriformis. Farnesane (“2,6,10-trimethyl dodecane”) neither promoted nor inhibited the growth (biomass) of fungi; nevertheless, the fuel user community should keep the strict care routineduring the storage of future mixtures with kerosene.
Supplementary information
(JPG 95.4 kb)
(JPG 75.6 kb)
(JPG 92.9 kb)
(JPG 49.3 kb)
(JPG 37.8 kb)
(JPG 47.2 kb)
Acknowledgements
The study was performed with the resources from LAB-BIO (Laboratory of Biodeterioration of Fuels and Biofuels) and the Post-Graduation Program in Agricultural and Environmental Microbiology of the Federal University of Rio Grande of Sul, Brazil. Mariane Lobato thanks the CAPES (Brazilian Federal Agency for Support and Evaluation of Graduate Education) for the Doctoral fellowship. We also thank Instituto Nacional de Tecnologia (INT), RBQAV (Rede Brasileira de Bioquerosene e Hidrocarbonetos Renováveis para aviação), Ubrabio (União Brasileira de Biodiesel e Bioquerosene), and a special reference to Pedro Rafael Scorza (in memorian) for his support and encouragement and for providing the farnesane sample.
Declarations
Competing interest
The authors declare no competing interests.
Footnotes
This paper is dedicated to the memory of Commander Pedro Rodrigo Scorza, who passed away.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chiaramonti D, Talluri G, Scarlat N, et al. The challenge of forecasting the role of biofuel in EU transport decarbonisation at 2050: a meta-analysis review of published scenarios. Renew Sustain Energy Rev. 2021;139:110715. doi: 10.1016/j.rser.2021.110715. [DOI] [Google Scholar]
- 2.Heyne J, Rauch B, Clerck PL, et al. Sustainable aviation fuel prescreening tools and procedures. Fuel. 2021;290:120004. doi: 10.1016/j.fuel.2020.120004. [DOI] [Google Scholar]
- 3.Hu D, Lin W, Zeng J, et al. (2020) Profiling the microbial contamination in aviation fuel from an airport. Biofouling. 2020;35:856–869. doi: 10.1080/08927014.2019.1671977. [DOI] [PubMed] [Google Scholar]
- 4.Ilha BKV, Santos LR, Santos LA, et al. Equações lineraes e não lineares para prever o comportamento de propriedades físico-químicas de combustível de aviação misturados com Bioquerosene Drop In alternativo. QuímicaNova. 2019;42:1–19. doi: 10.21577/0100-4042.20170302. [DOI] [Google Scholar]
- 5.Brown LM, McCombe JP, Vangness MD, et al. Community dynamics and phylogenetics of bacteria fueling Jet A and JP-8 aviation fuel. Int Biodeter Biodegr. 2010;64:253–261. doi: 10.1016/j.ibiod.2010.01.008. [DOI] [Google Scholar]
- 6.Krohn I, Bergmann L, Qi M, et al. Deep (Meta) genomics and (Meta) transcriptome analyses of fungal and bacteria consortia from aircraft tanks and kerosene identify key genes in fuel and tank corrosion. Front Microbiol. 2021;12:722250. doi: 10.3389/fmicb.2021.722259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin-Sanchez PM, Gorbushina AA, Kunte HJ, et al. A novel qPCR protocol for the specific detection and quantification of the fuel-deteriorating fungus Hormoconis resinae. Biofouling. 2016;32:635–644. doi: 10.1080/08927014.2016.1177515. [DOI] [PubMed] [Google Scholar]
- 8.Passman FJ. Microbial contamination and its control in fuels and fuel systems since1980- a review. Int Biodeter Biodegr. 2013;81:88–104. doi: 10.1016/j.ibiod.2012.08.002. [DOI] [Google Scholar]
- 9.Rauch M, Graef HW, Rozenzhak SM, et al. Characterization of microbial contamination in United States Air Force aviation fuel tanks. J Ind Microbiol Biotechnol. 2006;33:29–36. doi: 10.1007/s10295-005-0023-x. [DOI] [PubMed] [Google Scholar]
- 10.White J, Gilbert J, Hill G, et al. Culture-independent analysis of bacterial fuel contamination provides insight into the level of concordance with the standard industry practice of aerobic cultivation. Appl Environ Microbiol. 2011;77:4527–4538. doi: 10.1128/AEM.02317-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blakey S, Rye L, Wilson CW. Aviation gas turbine alternative fuels: a review. Proc Combust Inst. 2011;33:2863–2885. doi: 10.1016/j.proci.2010.09.011. [DOI] [Google Scholar]
- 12.Holladay J, Abdullah Z, Heyne J (2020) Sustainable aviation fuel: review of technical studies. Bioenergy Techno Off:1–81. 10.2172/1660415
- 13.Bakanaukas S (1958) Bacterial activity in JP-4 fuel. In: [S. l]: Wright Air Develop. Center, Rept 32-58. Defense Documentation Center No. AD 151034. US Air Force, Dayton, Ohio, USA
- 14.Gutheil NG, (1966) Ocorrênciade Cladosporium resinae (Lindau) de Vries em querosene de aviação no Brasil. Boletim N°. 9, InstitutoTecnológicodoRS,1966.Porto Alegre
- 15.Araya R, Bobadilla C, Rosalles V. Biochemical analysis of the Hormoconis resinae fungal mycelium in the corrosion of aeronautical aluminium alloys. RevistadeMetalurgia. 2007;43:228–236. doi: 10.3989/revmetalm.2007.v43.i3.68. [DOI] [Google Scholar]
- 16.Araya R, Bobadilla C, Rosalles V, Rosa V. Corrosion de aleaciones aeronauticas de aluminio y sus componentes relacionada a la expresion proteica del hongo hormoconis resinae. Inf Tecnol. 2008;19:59–68. doi: 10.4067/S0718-076420080002. [DOI] [Google Scholar]
- 17.Bento FM, Peralba MCR, Ferrão MF, Zimmer AR, et al. (2016) Diagnóstico, monitoramento e controle da contaminação microbiana em biodiesel e misturas durante o armazenamento. In:Pinho DMM, Suarez PAZ (Orgs) Armazenagem e uso de biodiesel: problemasassociados eformas decontrole.Brasília, p. 112-175.
- 18.Buddie AG, Bridge PD, Kelley J, et al. Candida kerosene esp. nov., a novel contaminantof aviationkerosene. Lett Appl Microbiol. 2011;52:70–75. doi: 10.1111/j.1472-765X.2010.02968.x. [DOI] [PubMed] [Google Scholar]
- 19.ChiciudeanI I, Mereuţă I, Ionescu R, et al. Jeta-1 bacterial contamination: a case study of cultivable bacteria diversity, alkane degradation and biofilm formation. Polish J Environ Stududies. 2019;28:4139–4146. doi: 10.15244/pjoes/99108. [DOI] [Google Scholar]
- 20.Climent E, Gotor R, Tobias C, et al. Dip sticks embedding molecular beacon-functionalized core-mesoporous shell particles for the rapidon-site detection of microbiological fuel contamination. Am Chem Soc. 2021;6:27–34. doi: 10.1021/acssensors.0c01178. [DOI] [PubMed] [Google Scholar]
- 21.Hill T. Microbial growth in aviation fuel. Aircraft Eng Aerospace Technol; Bradford. 2003;75:497–502. doi: 10.1108/00022660310492582. [DOI] [Google Scholar]
- 22.Leuchtle B, Epping L, Xie W, et al. Defined inoculum for the investigation of microbial contaminations of liquid fuels. Int Biodeter Biodegr. 2018;132:84–93. doi: 10.1016/j.ibiod.2017.05.017. [DOI] [Google Scholar]
- 23.Passman FJ, Kelley J, Whalen P. Interlaboratory study comparing two fuel microbiology standard test methods. Int Biodeter Biodegr. 2019;141:17–23. doi: 10.1016/j.ibiod.2018.07.005. [DOI] [Google Scholar]
- 24.Rafin C, Veignie E. Hormoconis resinae, the kerosene fungus. In: Mcgenity TJ, editor. Taxonomy, genomics and ecophysiology of hydrocarbon-degrading microbes. Springer, Switzerland: Handbook of Hydrocarbon and Lipid Microbiology; 2019. pp. 299–318. [Google Scholar]
- 25.Robbins JA, Lewy R (2004) A review of the microbial degradation of fuel. In: Paulus W (eds) Directory of microbicides for the protection of materials: a handbook. Springer, Dordrecht, Switzerland, pp177-201.
- 26.Rosales BM, Iannuzzi M. Aluminium AA2024T351 aeronautical alloy. Part 1. Microbial influenced corrosion analysis. Mater Sci Eng. 2008;472:15–25. doi: 10.1016/j.msea.2007.06.079. [DOI] [Google Scholar]
- 27.Valle SM. Tese (Doutorado em Engenharia de Minas, Metalúrgica e de Materiais) Porto Aelgre: Universidade Federal do Rio Grande do Sul; 1991. Estudo da corrosão microbiológica do alumínio e liga de alumínio 2024, porcontaminantesfúngicosdeturbocombustível. [Google Scholar]
- 28.Gaylarde CC, Bento FM, Kelley J. Microbial contamination of stored hydrocarbon fuels and its control. Rev Microbiol. 1999;30:1–10. doi: 10.1590/S0001-37141999000100001. [DOI] [Google Scholar]
- 29.Shapiro T, Chekanov K, Alexandrova A, et al. Revealing of non-cultivable bacteria associated with the mycelium of fungi in the kerosene-degrading community isolated from the contaminated jet fuel. J Fungy. 2021;7:43. doi: 10.3390/jof7010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Luo H, Yin X, et al. Surface coating on aluminum substrate with polymeric guanidine derivative to protect jet fuel tanks from microbial contamination. Surf Coat Technol. 2021;422:27521. doi: 10.1016/j.surfcoat.2021.127521. [DOI] [Google Scholar]
- 31.Hill EC, Hill GC. Microbialcontamination and associated corrosion in fuels, during storage, distribution and use. Adv Mat Res. 2008;38:257–268. doi: 10.4028/www.scientific.net/AMR.38.257. [DOI] [Google Scholar]
- 32.Silva TL, Cazarolli JC, Pizzolato TM, etal (2022) Microbial sludge formation in Brazilian marine diesel oil (B0) and soybean methylic biodiesel blends (B10 and B20) during simulated storage. Fuel 308: 121905. 10.1016/j.fuel.2021.121905
- 33.Hill GC. Investigation of Susceptibility of alternative jet fuels to microbiological growth: ALFA-BIRD project. St Mellons: ECHA Microbiology Limited; 2012. [Google Scholar]
- 34.Cazarolli JC, Guzzato R, Samios D, et al. Susceptibility of linseed, soybean, and olive biodiesel to growth of the deteriogenic fungus Pseudallescheria boydii. Int Biodeter Biodegr. 2014;95:364–372. doi: 10.1016/j.ibiod.2013.09.025. [DOI] [Google Scholar]
- 35.Cazarolli JC, Silva TL, Ribas RKC, etal (2020) Deterioration potential of Aureobasidium pullulans on biodiesel, diesel, and B20 blend. Int Biodeter Biodegr 147:104839 10.1016/j.ibiod.2019.104839
- 36.Ferreira ME, Grattapaglia D. Introdução ao uso de marcadores moleculares em análise genética. 3. Brasília: EMBRAPA-CENARGEN; 1996. [Google Scholar]
- 37.PETROBRÁS. Querosene de Aviação: informações técnicas. Rio de Janeiro. 2021. Disponível em:https://petrobras.com.br/data/files/9A/47/97/3E/104ED7105FC7BCD7E9E99EA8/Ma nual%20de%20Querosene%20de%20Aviacao%202021.pdf. Acesso em: 3 out. 2022.
- 38.National Agency of Petroleum Natural Gas and Biofuels - ANP Resolution N°. 846 of October 22, 2021 – DOU 10.25.2021. Regulates the specificationsof aviation fuels contained in ANP Technical Regulation No. 856/2021 and the obligations regarding quality control to be met by the various economic agents that market the product throughout the national territory. http://www.in.gov.br/en/web/DOU/ [access: 19 05.2023
- 39.Jiménez-Días L, Caballero A, Pérez-Hernández N, et al. Microbial alkane production for jet fuel industry: motivation, state of the art and perspectives. Microb Biotechnol. 2017;10:103–124. doi: 10.1111/1751-7915.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryder JA: Jet fuel compositions and methods of making and using same. United States Patent 2012, US 8106247 B2
- 41.Hanson KG, Desai JD, Desai AJ. A rapid and simple screening technique for potential crude oil degrading microorganisms. Biotechnol Tech. 1993;7:745–748. doi: 10.1007/BF00152624. [DOI] [Google Scholar]
- 42.Bushnell LD, Haas HFI. The utilization of hydrocarbons by microorganisms. J. Bacteriol. 1941;41:653–673. doi: 10.1128/jb.41.5.653-673.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lobato MR, Aranda DAG, Antoniosi Filho NRA et al (2020) Prospecção, identificação e capacidade de crescimento de microrganismos dequerosene de aviação e bioquerosene durante estocagem simulada.In: Nobre CP, Oliveira ACS (org) Estudosambientais e agronômicos, resultados para o Brasil, 1ed. Editora Pascal, São Luís:249–270
- 44.Ergin Ç, GöK Y, Bayĝu Y, et al. ATR-FTIR spectroscopy highlights the problem of distinguishing between Exophiala dermatitidis and E. phaeomuriformis using MALDI-TOF MS. Microb Ecol. 2016;71:339–346. doi: 10.1007/s00248-015-0670-z. [DOI] [PubMed] [Google Scholar]
- 45.Prenafeta-Boldú FX, Hoog GS, Summerbell RC. Fungal communities inhydrocarbon degradation. In: Mcgenity TJ, editor. Microbial communities utilizing hydrocarbons and lipids: members, metagenomics and ecophysiology. Switzerland: Springer Cham; 2019. pp. 307–342. [Google Scholar]
- 46.Isola D, Selbmann L, de Hoog SG, et al. Isolation and screening of black fungi as degraders of volatile aromatic hydrocarbons. Mycopathologia. 2013;175:369–379. doi: 10.1007/s11046-013-9635-2. [DOI] [PubMed] [Google Scholar]
- 47.SterflingerK . Black yeasts and meristematic fungi: ecology, diversity and identification. In: Peter G, Rosa C, editors. Biodiversity and ecophysiology of yeasts. Berlin: Springer; 2006. pp. 501–154. [Google Scholar]
- 48.Piontelli LE. Diversidad y polimorfismo enelgénero Exophiala: manejo de las especies comunesen el laboratorio de baja complejidad. BoletínMicológico. 2013;28:2–15. doi: 10.22370/bolmicol.2013.28.1.880. [DOI] [Google Scholar]
- 49.Sterflinger K. Fungi as geologic agents. Geomicrobiology. 2000;17:97–124. doi: 10.1080/01490450050023791. [DOI] [Google Scholar]
- 50.Zalar P, Nowak M, De Hoog SG, et al. Dishwashers-aman-madeecological niche accommodating human opportunistic fungal pathogens. Fungal Biol. 2011;115:997–1007. doi: 10.1016/j.funbio.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 51.Zupančič J, Raghupathi PK, Houf K, et al. Synergistic interactions in microbial biofilms facilitate the establishment of opportunistic pathogenic fungi in household dishwashers. Front Microbiol. 2018;9:113. doi: 10.3389/fmicb.2018.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matos T, Haase G, Gerrits AHG, et al. Molecular diversity of oligotrophic and neurotropic members of the black yeast genus Exophiala, with accenton E. dermatitidis. Antonie van Leeuwenhoek. 2003;83:293–303. doi: 10.1023/A:1023373329502. [DOI] [PubMed] [Google Scholar]
- 53.Najafzadeh MJ, Dolatabadi S, Keisari MS, et al. Detection and identification of opportunistic Exophiala species using the rolling circleamplification of ribosomal internal transcribed spacers. Journal of Microbiological Methods. 2013;94:338–342. doi: 10.1016/j.mimet.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 54.Siporin C, Cooney JJ. Inhibition of glucose metabolism by n hexadecane in Cladosporium (Amorphotheca) resinae. J Bacteriol. 1976;128:235–241. doi: 10.1128//jb.128.1.235-241.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miranda RC, Souza CS, Gomes EB, et al. Biodegradation of diesel oil by yeasts isolated from the vicinity of Suape Portin the State of Pernambuco -Brazil. Braz Arch Biol Technol. 2007;50:147–152. doi: 10.1590/S1516-89132007000100018. [DOI] [Google Scholar]
- 56.Junior JS, Mariano AP, Angelis DDF. Biodegradation of biodiesel/diesel blends by Candidavis wanathii. Afr J Biotechnol. 2009;8:2774–2778. doi: 10.5897/AJB09.238. [DOI] [Google Scholar]
- 57.de Souza LM, Mendes P, Aranda D. Assessing the current scenario of the Brazilian biojet market. Renew Sustain Energy Rev. 2018;98:426–438. doi: 10.1016/j.rser.2018.09.039. [DOI] [Google Scholar]
- 58.Parbery DG. The natural occurrence of Cladosporium resinae. Trans Br Mycol Soc. 1969;53:15–23. doi: 10.1016/S0007-1536(69)80002-1. [DOI] [Google Scholar]
- 59.Seifert KA, Hughes SJ, Boulay H, et al. Taxonomy, nomenclature and phylogeny of three cladosporium-like hyphomycetes, Sorocybere sinae, Seifertia azaleae and the Hormoconis anamorph of Amorphotheca resinae. Stud Mycol. 2007;58:235–245. doi: 10.3114/sim.2007.58.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Edmonds P, Cooney JJ. Identification of microorganisms isolated from jet fuelsystems. Appl Microbiol. 1967;15:411–416. doi: 10.1128/am.15.2.411-416.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guiamet PS, Saravia SGG. Biofilms formation and microbiologically influenced corrosion (MIC) in different materials. Innov Corros Mater Sci. 2017;7:2. [Google Scholar]
- 62.Martin-sanchez PM, Becker R, Gordushina AA, et al. Improved test for the evaluation of hydrocarbon degradation capacities of diesel-contaminating microorganisms. Int Biodeter Biodegr. 2018;129:89–94. doi: 10.1016/j.ibiod.2018.01.009. [DOI] [Google Scholar]
- 63.Raikos V, Vamvakas SS, Kapolos J, et al. (2011) Identification and characterization of microbial contaminants isolated from stored aviation fuels by DNA sequencing and restriction fragment length analysis of a PCR-amplifiedregion of the 16SrRNA gene. Fuel. 2011;90:695–700. doi: 10.1016/j.fuel.2010.09.030. [DOI] [Google Scholar]
- 64.Vidella HA, Guiamet PS, Valle S (1993) Effects of fungal and bacterial contaminants of kerosene fuels on the corrosion of storage and distribution systems. In: Kobrin G (Eds) A pratical manual on microbiologically influenced corrosion. NACE International, Houston, ppb125-139. 10.2174/2352094907666170420122727
- 65.Lindley ND, Heydeman MT. Alkane utilisation by Cladosporium resinae: the importance of extended lag phases when assessing substrate optima. FEMS Microbiol Lett. 1985;31:307–310. doi: 10.1111/j.1574-6968.1985.tb01164.x. [DOI] [Google Scholar]
- 66.Azambuja AO, Bücker F, Quadros PD, et al. Microbial community composition in Brazilian stored diesel fuel of varying sulfur content, using high-throughput sequencing. Fuel. 2017;189:340–349. doi: 10.1016/j.fuel.2016.10.108. [DOI] [Google Scholar]
- 67.Beker SA. Avaliação do potencial antimicrobiano de TBHQ (Terc-Butil-Hidroquinona) e de Bio-Óleo para uso em Biodiesel de soja (B100) e óleo diesel b (B10) Dissertação: Universidade Federal do Rio Grande do Sul; 2014. [Google Scholar]
- 68.Beker AS, Silva YP, Bücker F, et al. Effect of different concentrations of tert-butylhydroquinone (TBHQ) on microbial growth and chemical stability of soybean biodiesel during simulated storage. Fuel. 2016;84:701–707. doi: 10.1016/j.fuel.2016.07.067. [DOI] [Google Scholar]
- 69.Bento FM, Gaylarde CC. Biodeterioration of stored diesel oil: studies in Brazil. Int Biodeter Biodegr. 2001;47:107–112. doi: 10.1016/S0964-8305(00)00112-8. [DOI] [Google Scholar]
- 70.Bento FM, Camargo FAO, Okeke BC, et al. Bioremediation of soil contaminated by diesel oil. Braz J Microbiol. 2003;34:65–68. doi: 10.1590/S1517-8382200300050022. [DOI] [Google Scholar]
- 71.Bento FM, Camargo FAO, Okeke BC, et al. Diversity of biosurfactant producing microorganisms isolated from soils contaminated with diesel oil. Microbiol Res. 2005;160:249–255. doi: 10.1016/j.micres.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 72.Bücker F, Santestevan NS, Roesch LF, et al. Impact of biodiesel on biodeterioration of stored Brazilian diesel oil. Int Biodeter Biodegr. 2011;65:172–178. doi: 10.1016/j.ibiod.2010.09.008. [DOI] [Google Scholar]
- 73.Bücker F, Barbosa CS, PD Q, et al. Fuel biodegradation and molecular characterization of microbial biofilms in stored diesel/biodiesel blend B10 and the effect of biocide. Int Biodeter Biodegr. 2014;95:346–355. doi: 10.1016/j.ibiod.2014.05.030. [DOI] [Google Scholar]
- 74.CazarolliJC BF, Manique MC, et al. Suscetibilidade do biodiesel de sebo bovino à biodegradação por Pseudallescheria boydii. Revista Brasileira de Biociências. 2012;10:251–257. [Google Scholar]
- 75.Cazarolli JC, Quadros PD, Bücker F, etal (2016) Microbial growth in Acrocomia aculeata pulp oil, Jatropha curcas oil, and the irrespective biodiesels undersimulated storage conditions. Biofuel Res J 3: 514–520. 10.18331/BRJ2016.3.4.5
- 76.Cazarolli JC, Silva TL, Lobato MR, et al. Impact of water content on microbial growth in Brazilian biodiesel during simulated storage. Fuel. 2021;297:120761. doi: 10.1016/j.fuel.2021.120761. [DOI] [Google Scholar]
- 77.Cofone L, Walker JD, Cooney JJ. Utilization of hydrocarbonsby Cladosporium resinae. J Gen Microbiol. 1973;76:243–246. doi: 10.1099/00221287-76-1-243. [DOI] [PubMed] [Google Scholar]
- 78.Cooney JJ, Proby CM. Fatty acid composition of Cladosporium resinae grown on glucose and on hydrocarbons. J Bacteriol. 1971;108:777–718. doi: 10.1128/jb.108.2.777-781.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Teh JS, Lee KH. Utilization of n-alkanes by Cladosporium resinae. Appl Microbiol. 1973;25(3):454–457. doi: 10.1128/aem.25.3.454-457.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lindley ND, Heydeman MT. Mechanism of dodecane uptake by whole cells of Cladosporium resinae. J Gen Microbiol. 1986;132:751–756. doi: 10.1016/03-1097(85)90054-0. [DOI] [Google Scholar]
- 81.Boelter G, Cazarolli JC, Beker SA, et al. Pseudallescheria boydii and Meyerozyma guilliermondii: behavior of deteriogenic fungiduring simulated storage of diesel, biodiesel, and B10 blend in Brazil. Environ Sci Pollut Res. 2018;25:30410–30424. doi: 10.1007/s11356-018-3015-x. [DOI] [PubMed] [Google Scholar]
- 82.Bento FM, Gaylarde CC, Camargo FAO. Azevedo, JL, Melo, IS (Org) Microbiologia Ambiental. 2. Rio de Janeiro: Embrapa; 2008. Biossurfactantes; pp. 151–184. [Google Scholar]
- 83.Kaczorek E, Jesionowski T, Giec A, et al. Cell surface properties of Pseudomonas stutzeri in the process of diesel oil biodegradation. Biotechnol Lett. 2012;34:857–862. doi: 10.1007/s10529-011-0835-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang Y, Miller RM. Enhanced octadecane dispersion and biodegradation by a Pseudomonas rhamno lipid surfactant (biosurfactant) Appl Environ Microbiol. 1992;58:3276–3282. doi: 10.1128/aem.58.10.3576-3282.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Muriel JM, Bruque JM, Olías JM, et al. Production of biosurfactants by Cladosporium resinae. Microbiol Lett. 1996;18:235–240. [Google Scholar]
- 86.Floyd JG, Stamps BW, Bojanows CL et al (2022) The composition of diesel fuel influences the structure of microbiological assemblages in contaminated storage tanks. BioRxiv:1–41. 10.1101/2022.02.09.479836
- 87.Ruiz OM, Brown LM, Radwan O, et al. Metagenomic characterization reveals complex association of soil hydrocarbon-degrading bacteria. Int Biodeter Biodegr. 2021;157:105161. doi: 10.1016/j.ibiod.2020.105161. [DOI] [Google Scholar]
- 88.Smart C, Foley W, Ruiz OM et al (2012) Final report for the defense logistics agency (DLA) study of the effect of hydrocarbon type biodegradation on fuel specification properties, Dayton, OH 45469 N/A STU
- 89.Stamper DM, Morris RE, Montgomery MT. Depletion of lubricity improvers from hydrotreated renewable and ultra low-sulfur petroleum diesels by marine microbiota. Energy Fuels. 2012;26:6854–6862. doi: 10.1021/ef301158n. [DOI] [Google Scholar]
- 90.Sutton PA, Lewis CA, Rowland SJ. Isolation of individual hydrocarbons from the unresolved complex hydrocarbon mixture of a biodegraded crude oil using preparative capillary gas chromatography. Org Geochem. 2005;36:963–970. doi: 10.1016/j.orggeonchem.2004.11.007. [DOI] [Google Scholar]
- 91.Oh K-Bong, Woogchon M, Il-Moo C, (2001) Biodegradation of hidrocarbons by an organic solvent-tolerant fungus, Cladosporium resinae NK1. J Microbiol Biotechnol 11: 50-60. https://1017-7825(pISSN)/1738-8872
- 92.Maciel CCS, Souza CS, Silva PA, et al. Cinética de degradação de querosene de aviação por Penicillium sp. através da bioestimulação. Revista Brasileira de Biociências. 2013;11:39–42. [Google Scholar]
- 93.Khan SR, Nirmal JIK, Kumar RN, et al. Biodegradation of kerosene: study of growth optimization and metabolic fate of P. janthinellum SDX7. Braz J Microbiol. 2015;46:397–406. doi: 10.1590/S1517-838246220140112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lindley ND. Bioconversion and biodegradation of aliphatic hydrocarbons. Can J Bot. 1995;73:1034–1042. doi: 10.1139/b95-354. [DOI] [Google Scholar]
- 95.Walker JD, Cooney JJ. Pathway of n-alkane oxidation in Cladosporium resinae. J Bacteriol. 1973;115:635–639. doi: 10.1128/jb.115.2.635-639.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Goswami P, Cooney JJ. Subcellular location of enzymes involved in oxidation of n-alkane by Cladosporium resinae. Appl Environ Microbiol. 1999;51:860–864. doi: 10.1007/s002530051474. [DOI] [Google Scholar]
- 97.Hashen M, Alamri SA, Sharefah SAA, et al. Biodegradation and detoxification of aliphatic and aromatic hydrocarbons by new yeast strains. Ecotoxicol Environ Saf. 2018;151:28–34. doi: 10.1016/j.ecoenv.2017.12.064. [DOI] [PubMed] [Google Scholar]
- 98.MaierRMM . Microorganisms and organic pollutants. In: Pepper IL, Gerba CP, editors. Environmental microbiology. 2. San Diego: Academic Press; 2009. pp. 387–420. [Google Scholar]
- 99.Striebich RC, Smart C, Gunasekera TS, et al. Characterization of the F-76 diesel and Jet-A aviation fuel hydrocarbon degradation profiles of Pseudomonas aeruginosa and Marinobacter hydrocarbonoclasticus. Int Biodeter Biodegr. 2014;93:33–43. doi: 10.1016/j.ibiod.2014.04.024. [DOI] [Google Scholar]
- 100.Buijs NA, Siewers V, Nielsen J. Advanced biofuel production by the yeast Saccharomyces cerevisiae. Curr Opin Chem. Biol. 2013;17:480–488. doi: 10.1016/j.cbpa.2013.03.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(JPG 95.4 kb)
(JPG 75.6 kb)
(JPG 92.9 kb)
(JPG 49.3 kb)
(JPG 37.8 kb)
(JPG 47.2 kb)