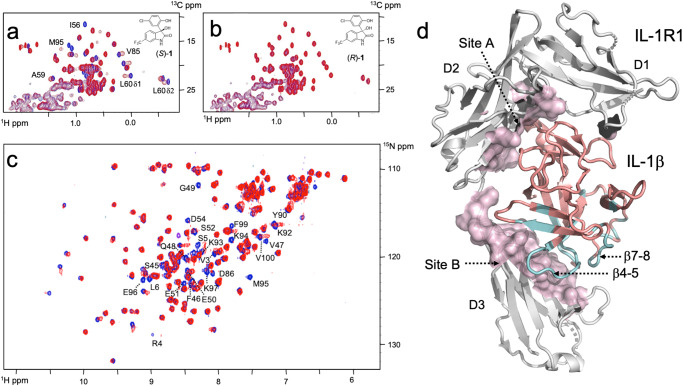
Fig. 1. Binding of fragment (1) to hIL-1β and mapping of its binding site.
a, b Superposition of 1H-13C-HMQC spectra for hIL-1β in the absence (blue) and presence of (S)-1 and (R)-1 (red). Residues that experience strong chemical shift changes upon the addition of (S)-1 are labeled. c Superposition of 1H–15N-HMQC spectra of hIL-1β in the absence (blue) and presence (red) of 1. Some residues that experience strong chemical shift differences or residues with intensities below the detection limit due to chemical exchange phenomena are labeled. d Residues that are affected upon binding of 1 to hIL-1β in 1H–15N HMQC spectra are mapped in cyan onto the X-ray structure of hIL-1β (PDB: 4DEP31). The affected residues cluster at the β-strands β1 and β5 and loops β4-5 and β7-8. Most of these residues are in close vicinity to domain 3 of the IL-1β/IL-1R1 interface, which represents site B of the interaction between IL-1β and its receptor.