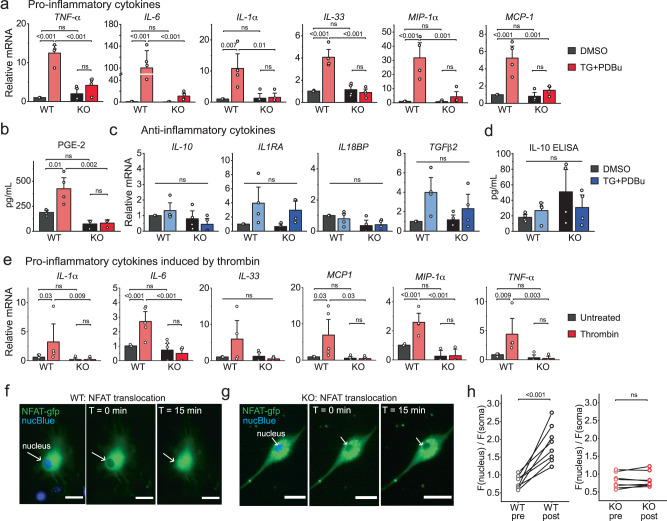
Fig. 4. Orai1 activation stimulates the production of pro-inflammatory cytokines from astrocytes.
a Induction of inflammatory cytokines and chemokines assessed by real-time PCR. WT (Orai1fl/fl) and Orai1 KO (Oraifl/fl GFAP-cre) astrocytes were stimulated for 6 h with a low dose (0.2 µM) of TG + 50 nM PDBu and cytokines were measured via two-step real-time PCR. mRNA levels were normalized to GAPDH. (n = 4 mice/group). b PGE-2 release from astrocytes measured by ELISA. (n = 4 WT mice, 3 KO mice.) c, d Anti-inflammatory cytokines assessed by qPCR (c) and ELISA (d) (n = 4 mice/group). e Induction of proinflammatory mediators by thrombin. Cytokines were measured by real-time PCR in astrocytes treated with thrombin (10 U/ml, 6 h) (n = 5 WT mice, 4 KO mice). f, g Activation of CRAC channels induces nuclear translocation of NFAT-GFP. WT (Orai1fl/fl) (f) or Orai1fl/fl GFAP-cre astrocytes (g) astrocytes were transfected with NFATc3-GFP to monitor NFAT dynamics. Cells were incubated with NucBlue for 20 min prior to imaging to visualize the nucleus. Cells were stimulated for 15 min with TG (1 µM) in 2 mM Ca2+ Ringer’s solution to stimulate Orai1 activity. h Quantification of NFAT-GFP nuclear translocation (ratio of the mean GFP intensity in the nuclear and cytosolic regions). Data are displayed as the nuclear/cell GFP fluorescence ratio (n = 8 cells from 3 mice/group). Scale bar = 10 µm. For all summary data, the bars indicate mean +/- SEM. For panel a: data separated by sex are provided in Supplementary Fig. 6. For panels b–h: the sex of the pups was not determined. Statistical tests were conducted by two-way ANOVA followed by Tukey posthoc tests for panels a–e, and paired t-test for panel h. Source data are provided as a Source Data file.