Fig. 2.
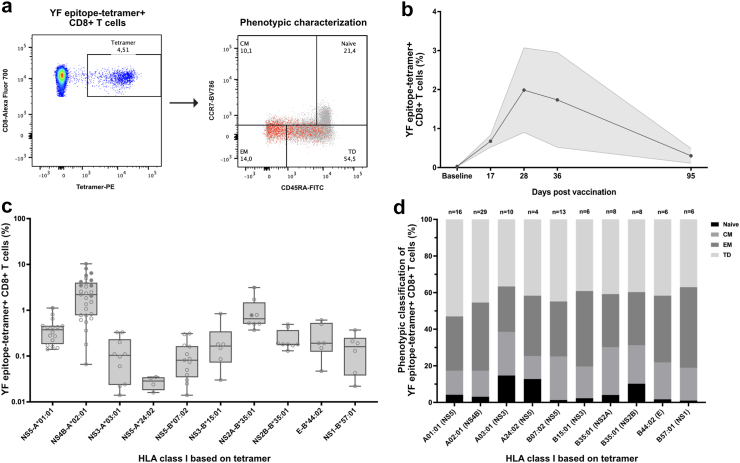
Induction of potent response of yellow fever-specific CD8+ T cells by YF-17D vaccination. a) Flow cytometry gating strategy for quantification and phenotypic characterization of YF-epitope tetramer + CD8+ T cells in PBMCs obtained from YF-17D vaccinated study participants post vaccination (21 ± 3 days). The phenotypic characterization flow plot depicts the subset distribution of YF-epitope tetramer + CD8+ T cells (red) across the total CD8+ T cells (grey) based on surface staining for CD45RA and CCR7: Naïve (CD45RA+, CCR7+), central memory (CM) (CD45RA-, CCR7+), effector memory (EM) (CD45RA-, CCR7-), and terminal differentiated (TD) (CD45RA+, CCR7-). b) YF-17D vaccination pilot study showing the frequency of YF-epitope tetramer + CD8+ T cells in total CD8+ T cells from YF-17D vaccinated individuals at baseline pre-vaccination and at day 17, 28, 36 and 95 post vaccination (n = 2, data shown as mean ± SEM). c) The frequency of YF-epitope tetramer + CD8+ T cells in total CD8+ T cells from 47 vaccinated study participants obtained post vaccination (21 ± 3 days). Data is plotted according to the donor HLA class I. Data for each individual donor is depicted with circles, and filled circles denotes values for individuals of which PBMCs are used in vitro cell killing assays (refer to Fig. 3, Fig. 4, Fig. 5). Box-and-whisker plot of the net frequency of YF-epitope tetramer + CD8+ T cells according to the donor HLA class I. d) Phenotypic characterization of YF epitope tetramer + CD8+T cells from 47 YF-17D vaccinated study participants. Each bar represents the mean subset composition of YF-epitope tetramer + CD8+ T cells in individuals with the HLA class I denoted below the bar. The number of individuals in each analysis is denoted above the bars.