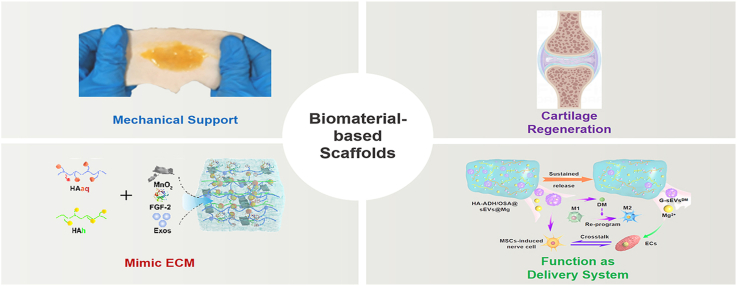
Abstract
Osteoarthritis (OA) poses a significant burden for countless individuals, inflicting relentless pain and impairing their quality of life. Although traditional treatments for OA focus on pain management and surgical interventions, they often fall short of addressing the underlying cause of the disease. Fortunately, emerging biomaterial-based scaffolds offer hope for OA therapy, providing immense promise for cartilage regeneration in OA. These innovative scaffolds are ingeniously designed to provide support and mimic the intricate structure of the natural extracellular matrix, thus stimulating the regeneration of damaged cartilage. In this comprehensive review, we summarize and discuss current landscape of biomaterial-based scaffolds for cartilage regeneration in OA. Furthermore, we delve into the diverse range of biomaterials employed in their construction and explore the cutting-edge techniques utilized in their fabrication. By examining both preclinical and clinical studies, we aim to illuminate the remarkable versatility and untapped potential of biomaterial-based scaffolds in the context of OA.
Thetranslational potential of this article
By thoroughly examining the current state of research and clinical studies, this review provides valuable insights that bridge the gap between scientific knowledge and practical application. This knowledge is crucial for clinicians and researchers who strive to develop innovative treatments that go beyond symptom management and directly target the underlying cause of OA. Through the comprehensive analysis and multidisciplinary approach, the review paves the way for the translation of scientific knowledge into practical applications, ultimately improving the lives of individuals suffering from OA and shaping the future of orthopedic medicine.
Keywords: Biomaterials, Cartilage, Osteoarthritis, RANKL, Regeneration, Scaffolds
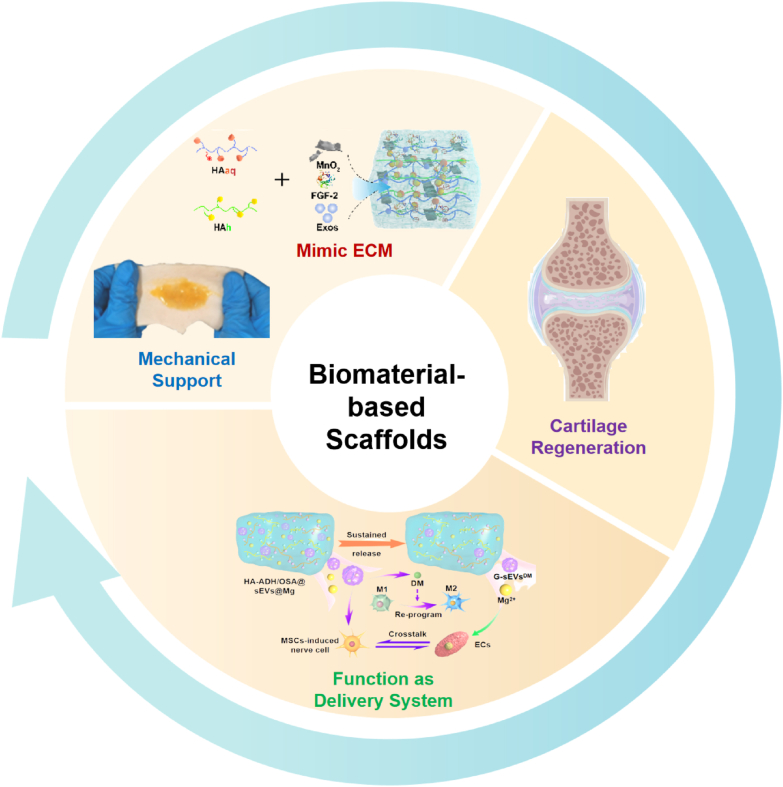
Graphical abstract
1. Introduction
Osteoarthritis (OA) is a prevalent degenerative joint disease characterized by the gradual damage of articular cartilage, along with changes in bone and soft tissues. It is influenced by various factors, including aging, obesity, genetic predisposition, and joint injuries [1]. Existing treatments for OA include non-pharmacological approaches like exercise and weight loss, as well as pharmacological therapies such as nonsteroidal anti-inflammatory drugs (NSAIDs) and analgesics [2]. However, these interventions primarily offer temporary relief from symptoms and do not directly target the fundamental degeneration of cartilage. Consequently, there is growing desire surrounding innovative regenerative strategies aimed at promoting the repair or replacement of impaired or diseased cartilage tissue.
Over the past few decades, the field of cartilage regeneration in OA has witnessed a significant progress with the utilization of biomaterial-based scaffolds [3]. These scaffolds possess an extraordinary capability to create a supportive three-dimensional (3D) environment that nurtures the growth and differentiation of cells, instilling hope for individuals suffering from OA [4]. By incorporating bioactive molecules like growth factors and cytokines into the scaffolds, researchers have unlocked the potential to enhance the regenerative processes within the body. Acting as messengers, these bioactive molecules signal the cells to undergo chondrogenesis, the process of cartilage formation, and drive tissue regeneration forward. Simultaneously, the scaffold provides necessary mechanical support for the development of structurally robust and functional cartilage tissue [4]. With the rapid advances in nanobiotechnology, biomaterial-based scaffolds hold tremendous promise for the field of cartilage regeneration in OA (Fig. 1). Their ability to construct a supportive 3D environment, mimic the native extracellular matrix (ECM), and enhance the power of bioactive molecules propels them to be widely used in regenerative medicine. However, there is a increasing need for further research to unravel the intricate molecular mechanisms that underlie the impact of biomaterial-based scaffolds on OA therapy. Moreover, our current knowledge remains inadequate in distinguishing the various biological activities and therapeutic effectiveness of scaffolds constructed using different biomaterials. These gaps emphasize the necessity for extensive investigations to deepen our comprehension of the biological properties and therapeutic potential of biomaterial-based scaffolds in the context of OA.
Fig. 1.
Schematic illustration of biomaterial-based scaffolds in promotion of cartilage regeneration. Reproduced with permission [5]. Copyright 2021, Wiley-VCH; reproduced with permission [6]. Copyright 2023, Wiley-VCH.
In this review, we provide a comprehensive overview of biomaterial-based scaffolds and their pivotal role in cartilage regeneration. First, we discuss the fundamental aspects of biomaterial-based scaffolds, shedding light on their essential features that make them indispensable in regenerative medicine. Furthermore, by exploration of the intricate interactions between biomaterials and cells, we highlight the eminent biocompatibility of these scaffolds, which fosters cellular adhesion, proliferation, and differentiation. Moreover, we elucidate the intricate mechanisms by which these scaffolds degrade, paving the way for the natural remodeling and regeneration of cartilage tissue. In addition, we shine a spotlight on the innovative techniques and technologies employed in creating scaffolds with precisely mediated properties. Finally, we thoroughly analyze the emerging studies to assess the tremendous potential of these scaffolds in restoring cartilage functionality and mitigating the debilitating effects of OA, emphasizing the urgent issues need to be overcome for widespread clinical translation.
2. Characteristics of biomaterial-based multifunctional scaffolds
The excellent advances in nanotechnology have ushered in a new era of possibilities, particularly in biomaterial-based scaffolds. The utilization of multifunctional scaffolds has opened up new horizons, offering a 3D framework that not only supports cell growth but also facilitates the regeneration of tissue. These scaffolds closely mimic the intricate ECM of the joint, fostering an environment that is conducive to cell growth and tissue regeneration [7]. In this section, we provide an in-depth exploration of the diverse properties exhibited by biomaterial-based scaffolds, underscoring their huge potential for cartilage regeneration in the context of OA.
Firstly, the success of their application in biomedicine depends on satisfying and beneficial biocompatibility and biodegradability [8]. Biocompatibility is a crucial property that indicates the scaffold's ability to interact safely with living tissues, without causing adverse effects such as toxicity, inflammation, or immune reactions. Additionally, biodegradability refers to the scaffold's capacity to slowly degrade and be metabolized by the body, facilitating the regeneration of new tissue. Among the wide range of materials used in multifunctional scaffolds, both natural and synthetic options have emerged as prominent choices [9].
Collagen and hyaluronic acid (HA) play a significant role in the development of biomaterial-based scaffolds, particularly due to their natural composition. These materials, present in the ECM of the joint, exhibit excellent biocompatibility and biodegradability, which support the growth of new tissue within the joint [10]. Leveraging their inherent properties, collagen and HA are ideal choices as they closely integrate with the surrounding tissues, providing an optimal environment for tissue regeneration.
In contrast, synthetic materials like PCL and poly(lactic-co-glycolic acid) (PLGA) have attracted accumulative interest in cartilage regeneration [11]. These materials can be functionally engineered to possess customized mechanical and structural properties, enabling tissue regeneration that closely resembles native tissue characteristics. PCL, a biodegradable polyester, demonstrates noteworthy biocompatibility and promotes cell adhesion and proliferation, thereby contributing to the success of tissue regeneration [12]. Similarly, PLGA, a copolymer of lactic acid and glycolic acid, offers both biodegradability and biocompatibility, further establishing itself as an exceptional candidate for cartilage regeneration [13].
The mechanical and structural properties of biomaterial-based scaffolds play a crucial role in their ability to restore damaged joint tissues [14]. These properties can be modulated by functionally adjusting the scaffold's composition, porosity, and shape [15]. Notably, the composition of the scaffold can be modulated to manipulate its mechanical and structural characteristics. For example, increasing the concentration of collagen or HA within the scaffold can enhance its stiffness and tensile strength [16]. Furthermore, porosity serves as an effective tool for modifying the scaffold's mechanical properties [17]. By increasing the pore size and volume fraction, the scaffold's stiffness can be reduced, promoting better integration with the surrounding tissue [18]. Additionally, the shape of the scaffold can be intelligently designed to optimize its mechanical properties, aligning with the unique shape of the joint to provide optimal support [19]. For instance, a cylindrical scaffold may offer superior mechanical reinforcement for a joint with a cylindrical shape [20].
More importantly, biomaterial-based scaffolds can be intelligently designed to possess targeted bioactive and functional properties [21]. By incorporating growth factors such as transforming growth factor-beta (TGF-β) or bone morphogenetic protein-2 (BMP-2) into the scaffold, the stimulation of chondrogenesis and osteogenesis, respectively, can be facilitated [22]. Furthermore, the surface topography of the scaffold can be functionally customized, offering a range of options from nanotopography to intricate micropatterns, which effectively promote cell adhesion and proliferation [22,23].
Moreover, the encapsulation of cells within the scaffold enhances its bioactivity and functionality. Mesenchymal stem cells (MSCs), known for their capacity to differentiate into chondrocytes and release bioactive molecules that promote tissue regeneration, offer great potential as a cell source for cartilage regeneration [24]. By seeding the scaffold with MSCs, not only can the regenerative capabilities of the scaffold be enhanced, but the mechanical properties of the regenerated tissue can also be improved.
Taken together, the combination of biocompatibility and biodegradability, mechanical and structural properties, and bioactivity and functionality collectively determines the success of biomaterial-based scaffolds in addressing OA. The capability to manipulate these properties empowers the development of customized biomaterials that foster the regeneration of tissue closely resembling native characteristics.
3. Recent advances in fabrication of biomaterial-based multifunctional scaffolds
The effectiveness of scaffolds in facilitating tissue regeneration depends on various critical factors, including structure, porosity, and fabrication method. In this section, we provide an overview of the most commonly utilized fabrication methods for scaffold-based biomaterials, focusing on additive manufacturing techniques, electrospinning, and decellularization and recellularization.
Additive manufacturing techniques, often known as 3D printing, have become an effective tool in producing scaffold-based biomaterials customized for cartilage regeneration [25]. The key advantage of 3D printing lies in its ability to exert precise control over scaffold structure and porosity, crucial for promoting tissue regeneration [26]. Additionally, this technique facilitates the fabrication of intricate geometries and shapes that were previously difficult to achieve using traditional fabrication methods [27].
Currently, a range of 3D printing techniques has been utilized in the fabrication of scaffold-based biomaterials, including fused deposition modeling (FDM), stereolithography (SLA), and selective laser sintering (SLS) [28]. FDM involves depositing layers of thermoplastic polymer onto a build platform to create the scaffold. SLA, on the other hand, employs a laser to cure a liquid resin, while SLS employs a laser to fuse powdered materials, often ceramics or metals, into the desired shape [29]. The choice of 3D printing technique depends on several factors, including the materials used, desired scaffold structure and porosity, and the intended application of the scaffold [29]. The choice of 3D printing technique depends on several factors, including the materials used, desired scaffold structure and porosity, and the intended application of the scaffold [30]. An important advantage of 3D printing is its ability to fabricate patient-specific scaffolds that are precisely tailored to the unique anatomy of the joint, potentially enhancing the effectiveness of the scaffold in promoting tissue regeneration.
Electrospinning is a valuable technique employed in the fabrication of nanofibrous scaffolds for cartilage regeneration [31]. This method involves applying a high voltage to a polymer solution, generating an electrostatic force that draws the polymer into fine, continuous fibers. These fibers are then collected on a collector, resulting in a scaffold with high surface area and porosity. Electrospun scaffolds offer numerous advantages for cartilage regeneration. The scaffold's high surface area and porosity facilitate efficient exchange of nutrients and oxygen, which are crucial for promoting tissue regeneration. Moreover, the nanofibrous structure of the scaffold closely resembles the native ECM of the joint, providing an ideal environment for cell growth and tissue regeneration [32]. Various polymers have been utilized in electrospinning, including PCL, PLGA, and poly(ethylene oxide) (PEO), with the choice depending on factors such as desired mechanical properties, biocompatibility, and biodegradability of the scaffold [33].
Decellularization and recellularization are widely used techniques that involve removing cellular components from tissues or organs, leaving behind the ECM as a scaffold. This ECM scaffold retains the native biochemical and mechanical properties of the tissue, making it invaluable for tissue regeneration [34]. Currently, these techniques have been successfully applied to promote the regeneration of various tissues, including cartilage, bone, and skin [35]. In the context of cartilage regeneration for OA, decellularized cartilage ECM serves as a scaffold for chondrocyte seeding and differentiation. The decellularization process typically involves the use of enzymes and detergents to eliminate cellular components while preserving the ECM. The resulting decellularized ECM scaffold can then be seeded with cells, either through direct injection or using a bioreactor, to promote tissue regeneration [36]. One significant advantage of decellularized ECM scaffolds is their ability to retain the native biochemical and mechanical properties of the tissue, which is crucial for promoting tissue regeneration [37]. Additionally, using autologous cells derived from the patient's own tissue holds the potential to enhance the scaffold's effectiveness in promoting tissue regeneration while reducing the risk of rejection [38]. However, the use of decellularized ECM scaffolds also has limitations. Achieving complete decellularization remains challenging, as residual cellular components may trigger immune responses and hinder tissue regeneration. Moreover, reliance on a reliable source of donor tissue can be limiting, as the availability of such tissue may be restricted in certain cases.
4. Emerging applications of biomaterial-based scaffolds in OA treatments
The utilization of biomaterials as delivery vehicles represents a promising strategy for enhancing the efficacy of therapeutic agents in the treatment of OA. This approach enables the sustained release of therapeutic agents, mitigates systemic toxicity, and improves local concentrations precisely at the site of injury [39]. In this section, we introduce various approaches employed for the delivery of therapeutic agents using biomaterial-based scaffolds in OA therapy.
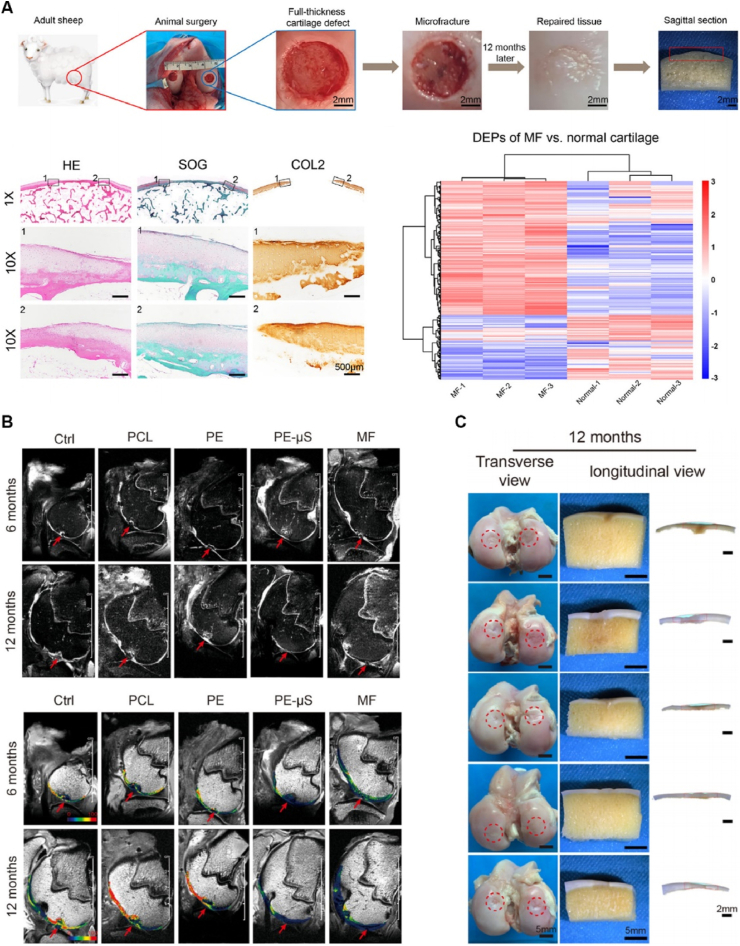
Growth factors serve as vital signaling molecules that orchestrate tissue regeneration by stimulating cell proliferation, migration, and differentiation. The delivery of growth factors in conjunction with biomaterial-based scaffolds holds the potential to augment their local concentrations, facilitating the sustained release of therapeutic agents precisely at the site of injury (Fig. 2) [40]. Numerous growth factors have been extensively investigated for their role in cartilage regeneration, including TGF-β, BMP-2, and insulin-like growth factor-1 (IGF-1) [41]. One approach employed for growth factor delivery involves the use of growth factor-loaded microspheres or nanoparticles that are incorporated into the scaffold. These particles act as reservoirs, ensuring the sustained release of the growth factor, thus enhancing its local concentrations at the site of injury [42]. Another approach entails the covalent immobilization of the growth factor onto the scaffold surface. This method enables the sustained release of the therapeutic agent over an extended period, providing a continuous supply of the growth factor at the site of injury [43].
Fig. 2.
(A) Schematic illustration of the use of growth factor-encapsulated scaffolds for treating cartilage defects in adult sheep. (B) Representative T2 mapping images of different groups. (C) Representative images of the repaired tissues at 12 months postsurgery. Reproduced with permission from Ref. [40]. Copyright 2022, Elsevier.
The incorporation of growth factor-loaded microspheres or nanoparticles within the scaffold offers considerable advantages. Firstly, it facilitates a controlled and sustained release of the growth factor, ensuring a prolonged presence at the site of injury. This sustained delivery promotes optimal cell responses and tissue regeneration over an extended period [6,44,45]. Furthermore, it allows for precise control over the release kinetics, mediating the delivery to meet the specific requirements of the regenerative process. Moreover, the incorporation of microspheres or nanoparticles within the scaffold offers protection to the growth factor, shielding it from degradation and enhancing its stability [46,47]. This protective effect ensures the bioactivity of the growth factor during the release process, thereby maximizing its therapeutic potential.
Alternatively, the covalent immobilization of growth factors onto the scaffold surface presents its own set of advantages [48]. By attaching the growth factor directly to the scaffold, a sustained release of the therapeutic agent can be achieved over a more prolonged duration [48]. The covalent bonding ensures the stability of the growth factor and prevents its premature release. Moreover, this approach allows for greater control over the release kinetics by modifying the nature and density of the covalent attachment [49]. This versatility enables the tailoring of the release profile to meet the specific needs of the regenerative process.
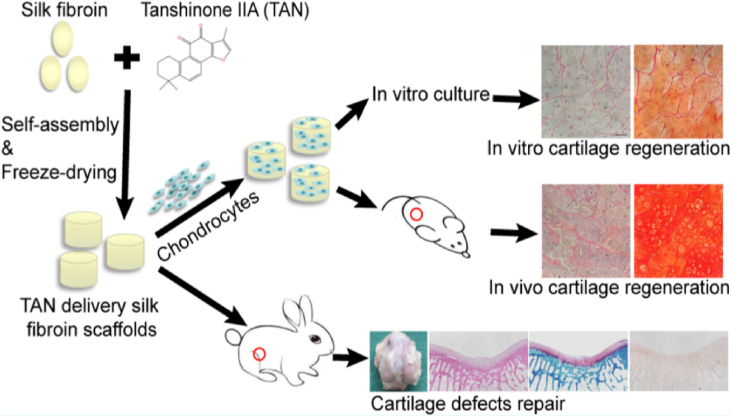
Drugs such as NSAIDs and corticosteroids have long been employed in the treatment of OA. However, recent studies have shed light on the promising potential of scaffold-based drug delivery systems to enhance the therapeutic efficacy for OA. These emerging findings have provided new avenues for more effective treatments. One noteworthy study explored the use of a silk fibroin scaffold incorporated with tanshinone IIA (TAN) for promoting cartilage regeneration. This innovative approach demonstrated beneficial outcomes, as the TAN10 delivery silk fibroin scaffolds exhibited a significant enhancement in the generation of cartilage-specific ECM by chondrocytes compared to conventional silk fibroin scaffolds. This eminent discovery offers a promising strategy for future treatment of cartilage defects (Fig. 3) [50].
Fig. 3.
Schematic illustration of TAN10 delivery silk fibroin scaffolds in treating cartilage defects. Reproduced with permission from Ref. [50]. Copyright 2020, American Chemical Society.
Generally, two primary methods have been employed in the fabrication of drug-delivery scaffolds. The first involves incorporating drug-loaded microspheres or nanoparticles into the scaffold, allowing for controlled and sustained release of the therapeutic drug over the course of treatment. The second method entails the covalent immobilization of the drug onto the surface of the scaffold. Both approaches aim to ensure a sustained release pattern of the therapeutic drug during the treatment period, maximizing its effectiveness [51].
These advances in drug-delivery scaffolds represent a significant step forward in the field of OA treatment. By providing a sustained and targeted release of therapeutic drugs, these scaffolds hold great promise for improving patient outcomes and addressing the unmet needs in the management of cartilage defects.
Gene therapy holds tremendous promise as a cutting-edge approach in the treatment of OA. By enabling sustained expression of therapeutic genes, such as growth factors, anti-inflammatory agents, and chondrogenic factors, it offers the potential for long-lasting and targeted interventions. To enhance the delivery of genes to specific cells, biomaterial-based scaffolds have emerged as a valuable tool, facilitating efficient gene delivery and promoting the proliferation and migration of chondrocytes. This, in turn, can significantly improve the therapeutic efficacy of OA [52].
An encouraging study conducted by Yang et al. exemplifies the potential of combining gene therapy with tissue engineering methods to enhance cartilage repair. Their research demonstrated promising results both in vitro and in vivo, showing the effectiveness of gene-modified biomaterial-based scaffolds as a strategy for treating cartilage lesions [53]. This innovative approach represents a significant advance in cartilage regeneration, offering new possibilities for more targeted and effective treatments.
Furthermore, Capito et al. reported a novel strategy by developing a type II collagen-glycosaminoglycan (CG) scaffold specifically designed to induce chondrogenesis and enhance cartilage regeneration [43]. By acting as a vehicle for gene delivery, this scaffold facilitates enhanced, prolonged, and localized expression of IGF-1, a key regulator in promoting cartilage regeneration [43]. This pioneering research demonstrates the potential of biomaterial-based scaffolds not only as carriers for therapeutic genes but also as agents that actively contribute to the regenerative process.
Moreover, non-coding RNAs, including microRNAs and long non-coding RNAs, have emerged as highly promising therapeutic targets for OA. These RNAs possess the excellent ability to regulate gene expression and modulate crucial cellular processes [54]. Another potent therapeutic agent, small interfering RNA (siRNA), holds great potential for OA treatment as well, as it can precisely target specific genes implicated in the pathogenesis of the disease. The delivery of non-coding RNAs and siRNA utilizing biomaterial-based scaffolds offers a safe and effective means to transport the active molecules to target cells, playing a significant role in enhancing cartilage regeneration. For instance, Chen et al. devised and utilized a nanoparticle-encapsulated hydrogel scaffold for the delivery of siRNA, effectively inhibiting the infiltration of the local vascular system and bolstering cartilage regeneration. The in vitro and in vivo findings demonstrated that this scaffold achieved superior cartilage regeneration through sustained release of siRNA, resulting in the upregulation of essential factors such as SOX9, COL-II, and ACAN [55]. This study indicates the potential of biomaterial-based scaffolds in delivering siRNA, thereby enabling targeted gene regulation and promoting the restoration of healthy cartilage.
In addition to RNA-based therapies, cell therapy has emerged as a highly promising approach for OA treatment, with biomaterial-based scaffolds serving as key facilitators in enhancing the efficacy of cell-based therapies [56]. Cells can be delivered to the affected site either through direct seeding onto the scaffold or by incorporating cells into the scaffold during the fabrication process. The use of scaffold-based biomaterials provides a conducive 3D microenvironment for optimal cell growth and differentiation, thereby promoting the formation of functional tissue [57].
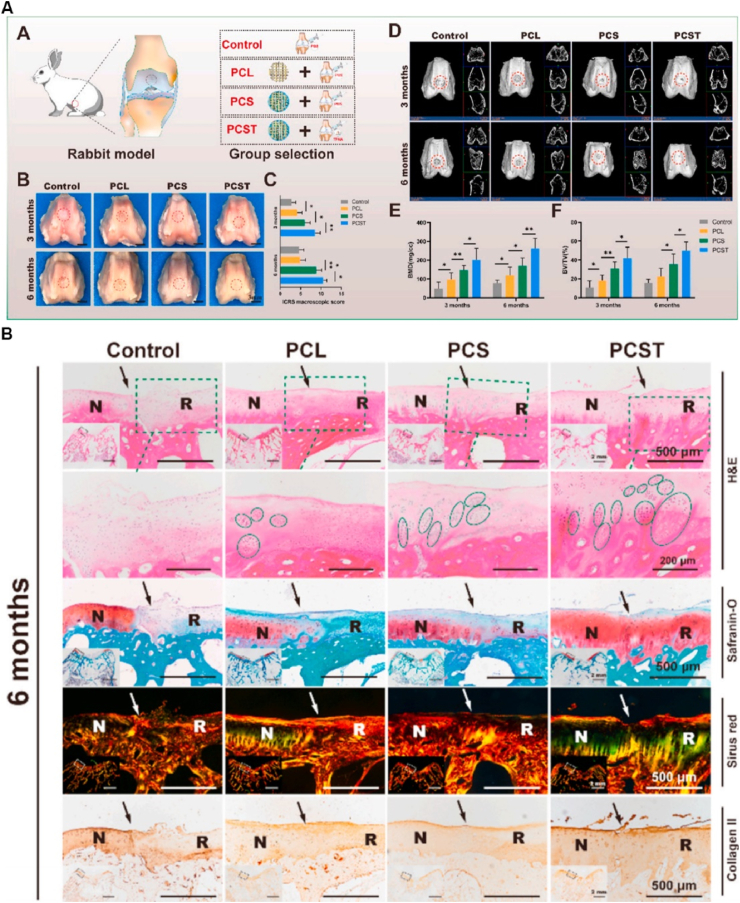
One of the challenges faced in cell-based therapies is the limited survival and retention of transplanted cells. However, biomaterial-based scaffolds play a crucial role in improving cell survival and retention by providing a supportive microenvironment for cell growth and proliferation [58]. Moreover, these scaffolds can facilitate cell differentiation by offering mechanical and biochemical cues that closely mimic the native tissue environment [59]. Numerous studies have explored the potential of scaffold-based biomaterials for cell delivery in cartilage regeneration [60]. For example, Li et al. developed a 3D-printed polycaprolactone (PCL) scaffold that was seeded with MSCs for the treatment of OA. Their results demonstrated that the scaffold-based biomaterials significantly enhanced the chondrogenic differentiation of MSCs and promoted the formation of cartilage tissue both in vitro and in vivo (Fig. 4) [12].
Fig. 4.
(A) Group design, macroscopic evaluation and micro-CT analysis of the experimental rabbits. (B) Histological and immunohistochemical examination of the defect area at 6 months potsurgery. Reproduced with permission from Ref. [12]. Copyright 2021, Elsevier.
These advances in utilizing biomaterial-based scaffolds in conjunction with non-coding RNAs, siRNA, and cell therapies represent significant advances in OA treatment. By harnessing the potential of these innovative approaches, researchers are paving avenue for more targeted and effective treatments that hold great promise in combating this debilitating condition and improving patients’ quality of life.
Of note, exosomes, small extracellular vesicles secreted by cells, play a crucial role in facilitating cell-to-cell communication [6]. These remarkable vesicles carry a cargo of proteins, lipids, and nucleic acids, including miRNAs and other non-coding RNAs, which possess the ability to regulate gene expression in recipient cells [61]. Recently, exosomes have emerged as a promising avenue for delivering therapeutic molecules, such as growth factors and miRNAs, for the treatment of OA [62].
The integration of biomaterial-based scaffolds has proven instrumental in enhancing the delivery and retention of exosomes within joint tissues. Exosomes can be incorporated into the scaffold during fabrication or loaded onto the scaffold after its construction. The utilization of biomaterial-based scaffolds serves to shield exosomes from degradation and improve their retention in the joint tissue, thereby maximizing their therapeutic efficacy [63].
In a notable study conducted by Zhang et al., an injectable, highly adhesive hydrogel inspired by mussel proteins was developed in combination with exosomes to investigate their potential for endogenous cell recruitment and cartilage defect regeneration [64]. The findings demonstrated that the biomaterial-based scaffolds greatly enhanced the retention and therapeutic efficacy of exosomes in the joint tissue. This innovative approach not only promoted the regeneration of cartilage tissue but also contributed to a reduction in inflammation, showing the transformative potential of biomaterial-based scaffolds in utilizing the therapeutic capabilities of exosomes. By leveraging the unique properties of exosomes and incorporating them into scaffolds, researchers are unlocking new possibilities for targeted and efficient delivery of therapeutic molecules.
5. Challenges and future directions
The fabrication of biomaterial-based scaffolds for cartilage regeneration poses a significant challenge in creating a microenvironment that can beneficially mimic the natural ECM of cartilage tissue. The scaffold should fulfill multiple functions, including providing mechanical support, facilitating cell adhesion, proliferation, and differentiation, as well as promoting chondrogenesis [65,66]. Furthermore, it is crucial for the scaffold to exhibit good biocompatibility and biodegradability, and appropriate mechanical and degradation properties. Striking a balance between these diverse requirements can be a complex task that necessitates meticulous material selection and optimization.
Another noteworthy hurdle lies in the development of strategies to enhance the integration of the scaffold with the surrounding tissue. Insufficient integration can lead to implant failure, inflammation, and immune responses [67]. Various techniques have been proposed to address this challenge, including surface modification, incorporation of adhesion peptides, and the utilization of growth factors and cytokines [68]. Furthermore, the absence of an ideal cell source for cartilage regeneration poses a significant limitation in the context of biomaterial-based scaffolds for OA therapy. Although multiple cell types, such as chondrocytes, mesenchymal MSCs, and induced pluripotent stem cells (iPSCs), have been explored for cartilage regeneration, each has its own limitations, ranging from low cell viability and dedifferentiation to ethical concerns [69]. The safety and regulatory aspects associated with biomaterial-based scaffolds for cartilage regeneration parallel those of other biomaterials. Additionally, potential risks linked to scaffold implantation, including infection, inflammation, and tissue damage, should be thoroughly evaluated.
Although biomaterial-based scaffolds hold significant promise for cartilage regeneration in the treatment of OA, they are not without potential drawbacks and challenges. Three important aspects to consider are immunological responses, mechanical properties, and long-term durability [70,71]. When using biomaterial-based scaffolds, one concern is the potential for immunological responses in the body [3]. The presence of foreign materials can trigger an immune reaction, leading to inflammation and potential rejection of the scaffold. The immune response can hinder the effectiveness of the scaffold and impede tissue regeneration. Furthermore, the mechanical properties of biomaterial-based scaffolds play a crucial role in their ability to support and restore joint function. The scaffold should possess adequate strength, stiffness, and resilience to withstand physiological forces and maintain structural integrity [63]. Insufficient mechanical properties can result in poor load-bearing capacity and may compromise the long-term success of the scaffold. Another consideration is the long-term durability and stability of the scaffold [71]. Over time, the scaffold may experience degradation, loss of structural integrity, or changes in its mechanical properties. The degradation process should align with the rate of tissue regeneration to maintain the scaffold's support and functionality throughout the healing process. Achieving the right balance between scaffold degradation and tissue regeneration is critical to ensure long-term durability and effectiveness [72]. To expedite the translation of these biomaterials from laboratory research to practical applications, several recommendations can be proposed.
-
(i)
Advancement of new and improved biomaterials that possess enhanced mechanical, degradation, and biocompatibility properties.
-
(ii)
Identification of suitable cell sources and optimization of protocols for cell-based cartilage regeneration.
-
(iii)
Integration of advanced technologies, such as 3D printing and bioprinting, to fabricate intricate and patient-specific scaffolds.
-
(iv)
Development of strategies to bolster scaffold integration and tissue regeneration, such as the utilization of growth factors, cytokines, and exosomes.
-
(v)
Implementation of well-designed preclinical and clinical studies to rigorously evaluate the safety and efficacy of scaffold-based biomaterials for OA therapy.
-
(vi)
Collaboration among academia, industry, and regulatory agencies to foster the seamless translation of scaffold-based biomaterials from the research bench to clinical implementation.
By addressing these recommendations and overcoming the existing challenges, the field of biomaterial-based scaffolds for OA therapy can realize its full potential in revolutionizing the treatment of this debilitating condition and improving the lives of OA patients.
6. Conclusions
In summary, the field of biomaterial-based scaffolds for cartilage regeneration is rapidly advancing, which has the potential to revolutionize the treatment of OA and significantly impact the lives of millions of patients worldwide. Future efforts should focus on developing scaffolds with enhanced biocompatibility and biodegradability to ensure compatibility with the physiological environment. Optimizing delivery methods for growth factors and other bioactive molecules is another crucial area for exploration, as it can significantly enhance the therapeutic efficacy of these scaffolds. Additionally, integrating novel cell-based and tissue engineering strategies will further enhance the regenerative potential of these scaffolds. While challenges persist, sustained research and development efforts in this field provide considerable potential for the future of OA therapy. With continued advancements, biomaterial-based scaffolds may become a fundamental aspect of effective and transformative treatments for OA, ultimately improving the quality of life for individuals grappling with this degenerative joint disease.
Author contributions
Conceptualization, H.Z.; writing—original draft preparation, J.L. and P.L.; writing—review and editing, X.Y. and L.L.; visualization, Y.Z. and Q.W. All authors have read and agreed to the published version of the manuscript.
Funding
This review was funded by Scientific Research Project of Xi'an Jiaotong University (xzy012022130) and Xi'an Talent Plan Project (XAYC210060).
Data availability statement
Not applicable.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
We would like to thank Figdraw software (http://www.Figdraw.com) for its picture source assistance.
References
- 1.Jiang Y. Osteoarthritis year in review 2021: biology. Osteoarthritis Cartilage. 2022;30(2):207–215. doi: 10.1016/j.joca.2021.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Abramoff B., Caldera F.E. Osteoarthritis: pathology, diagnosis, and treatment options. Med Clin. 2020;104(2):293–311. doi: 10.1016/j.mcna.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Klimak M., Nims R.J., Pferdehirt L., Collins K.H., Harasymowicz N.S., Oswald S.J., et al. Immunoengineering the next generation of arthritis therapies. Acta Biomater. 2021;133:74–86. doi: 10.1016/j.actbio.2021.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shalumon K.T., Chen J.P. Scaffold-based drug delivery for cartilage tissue regeneration. Curr Pharmaceut Des. 2015;21(15):1979–1990. doi: 10.2174/1381612821666150302152836. [DOI] [PubMed] [Google Scholar]
- 5.Xiong Y., Chen L., Liu P., Yu T., Lin C., Yan C., et al. All-in-One: multifunctional hydrogel accelerates oxidative diabetic wound healing through timed-release of exosome and fibroblast growth factor. Small. 2022;18(1) doi: 10.1002/smll.202104229. [DOI] [PubMed] [Google Scholar]
- 6.Xiong Y., Lin Z., Bu P., Yu T., Endo Y., Zhou W., et al. A whole-course-repair system based on Neurogenesis-angiogenesis crosstalk and macrophage reprogramming promotes diabetic wound healing. Adv Mater. 2023;35(19) doi: 10.1002/adma.202212300. [DOI] [PubMed] [Google Scholar]
- 7.Toh W.S., Spector M., Lee E.H., Cao T. Biomaterial-mediated delivery of microenvironmental cues for repair and regeneration of articular cartilage. Mol Pharm. 2011;8(4):994–1001. doi: 10.1021/mp100437a. [DOI] [PubMed] [Google Scholar]
- 8.Lopa S., Mondadori C., Mainardi V.L., Talo G., Costantini M., Candrian C., et al. Translational application of microfluidics and bioprinting for stem cell-based cartilage repair. Stem Cell Int. 2018;2018 doi: 10.1155/2018/6594841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frassica M.T., Grunlan M.A. Perspectives on synthetic materials to guide tissue regeneration for osteochondral defect repair. ACS Biomater Sci Eng. 2020;6(8):4324–4336. doi: 10.1021/acsbiomaterials.0c00753. [DOI] [PubMed] [Google Scholar]
- 10.Iulian A., Dan L., Camelia T., Claudia M., Sebastian G. Synthetic materials for osteochondral tissue engineering. Adv Exp Med Biol. 2018;1058:31–52. doi: 10.1007/978-3-319-76711-6_2. [DOI] [PubMed] [Google Scholar]
- 11.Kalkan R., Nwekwo C.W., Adali T. The use of scaffolds in cartilage regeneration. Crit Rev Eukaryot Gene Expr. 2018;28(4):343–348. doi: 10.1615/CritRevEukaryotGeneExpr.2018024574. [DOI] [PubMed] [Google Scholar]
- 12.Li P., Fu L., Liao Z., Peng Y., Ning C., Gao C., et al. Chitosan hydrogel/3D-printed poly(epsilon-caprolactone) hybrid scaffold containing synovial mesenchymal stem cells for cartilage regeneration based on tetrahedral framework nucleic acid recruitment. Biomaterials. 2021;278 doi: 10.1016/j.biomaterials.2021.121131. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y., Yang F., Liu K., Shen H., Zhu Y., Zhang W., et al. The impact of PLGA scaffold orientation on in vitro cartilage regeneration. Biomaterials. 2012;33(10):2926–2935. doi: 10.1016/j.biomaterials.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Rezus E., Burlui A., Cardoneanu A., Macovei L.A., Tamba B.I., Rezus C. From pathogenesis to therapy in knee osteoarthritis: bench-to-bedside. Int J Mol Sci. 2021;22(5) doi: 10.3390/ijms22052697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armiento A.R., Stoddart M.J., Alini M., Eglin D. Biomaterials for articular cartilage tissue engineering: learning from biology. Acta Biomater. 2018;65:1–20. doi: 10.1016/j.actbio.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 16.Newham G., Evans S.D., Ong Z.Y. Mechanically tuneable physical nanocomposite hydrogels from polyelectrolyte complex templated silica nanoparticles for anionic therapeutic delivery. J Colloid Interface Sci. 2022;617:224–235. doi: 10.1016/j.jcis.2022.02.052. [DOI] [PubMed] [Google Scholar]
- 17.Cheng L., Tong X., Li Z., Liu Z., Huang H., Zhao H., et al. Natural silkworm Cocoon composites with high strength and stiffness constructed in confined cocooning space. Polymers. 2018;10(11) doi: 10.3390/polym10111214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciritsis A., Horbach A., Staat M., Kuhl C.K., Kraemer N.A. Porosity and tissue integration of elastic mesh implants evaluated in vitro and in vivo. J Biomed Mater Res B Appl Biomater. 2018;106(2):827–833. doi: 10.1002/jbm.b.33877. [DOI] [PubMed] [Google Scholar]
- 19.Skotak M., Noriega S., Larsen G., Subramanian A. Electrospun cross-linked gelatin fibers with controlled diameter: the effect of matrix stiffness on proliferative and biosynthetic activity of chondrocytes cultured in vitro. J Biomed Mater Res. 2010;95(3):828–836. doi: 10.1002/jbm.a.32850. [DOI] [PubMed] [Google Scholar]
- 20.Foroughi A.H., Razavi M.J. Multi-objective shape optimization of bone scaffolds: enhancement of mechanical properties and permeability. Acta Biomater. 2022;146:317–340. doi: 10.1016/j.actbio.2022.04.051. [DOI] [PubMed] [Google Scholar]
- 21.Fan S., Chen K., Yuan W., Zhang D., Yang S., Lan P., et al. Biomaterial-based scaffolds as antibacterial suture materials. ACS Biomater Sci Eng. 2020;6(5):3154–3161. doi: 10.1021/acsbiomaterials.0c00104. [DOI] [PubMed] [Google Scholar]
- 22.Raina D.B., Qayoom I., Larsson D., Zheng M.H., Kumar A., Isaksson H., et al. Guided tissue engineering for healing of cancellous and cortical bone using a combination of biomaterial based scaffolding and local bone active molecule delivery. Biomaterials. 2019;188:38–49. doi: 10.1016/j.biomaterials.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Madhusoodan A.P., Das K., Mili B., Kumar K., Kumar A., Saxena A.C., et al. In vitro proliferation and differentiation of canine bone marrow derived mesenchymal stem cells over hydroxyl functionalized CNT substrates. Biotechnol Rep (Amst) 2019;24 doi: 10.1016/j.btre.2019.e00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang B., Chariyev-Prinz F., Burdis R., Eichholz K., Kelly D.J. Additive manufacturing of cartilage-mimetic scaffolds as off-the-shelf implants for joint regeneration. Biofabrication. 2022;14(2) doi: 10.1088/1758-5090/ac41a0. [DOI] [PubMed] [Google Scholar]
- 25.Daly A.C., Freeman F.E., Gonzalez-Fernandez T., Critchley S.E., Nulty J., Kelly D.J. 3D bioprinting for cartilage and osteochondral tissue engineering. Adv Healthcare Mater. 2017;6(22) doi: 10.1002/adhm.201700298. [DOI] [PubMed] [Google Scholar]
- 26.Wang S., Zhao S., Yu J., Gu Z., Zhang Y. Advances in translational 3D printing for cartilage, bone, and osteochondral tissue engineering. Small. 2022;18(36) doi: 10.1002/smll.202201869. [DOI] [PubMed] [Google Scholar]
- 27.Shi W., Sun M., Hu X., Ren B., Cheng J., Li C., et al. Structurally and functionally optimized silk-fibroin-gelatin scaffold using 3D printing to repair cartilage injury in vitro and in vivo. Adv Mater. 2017;29(29) doi: 10.1002/adma.201701089. [DOI] [PubMed] [Google Scholar]
- 28.Su X., Wang T., Guo S. Applications of 3D printed bone tissue engineering scaffolds in the stem cell field. Regen Ther. 2021;16:63–72. doi: 10.1016/j.reth.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen S., Chen M., Guo W., Li H., Li X., Huang S., et al. Three dimensional printing-based strategies for functional cartilage regeneration. Tissue Eng Part B Rev. 2019;25(3):187–201. doi: 10.1089/ten.TEB.2018.0248. [DOI] [PubMed] [Google Scholar]
- 30.Xue J., Qin C., Wu C. 3D printing of cell-delivery scaffolds for tissue regeneration. Reg Biomater. 2023;10 doi: 10.1093/rb/rbad032. rbad032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Mori A., Pena Fernandez M., Blunn G., Tozzi G., Roldo M. 3D printing and electrospinning of composite hydrogels for cartilage and bone tissue engineering. Polymers. 2018;10(3) doi: 10.3390/polym10030285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Y. Electrohydrodynamic jet 3D printing in biomedical applications. Acta Biomater. 2021;128:21–41. doi: 10.1016/j.actbio.2021.04.036. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y., Zhang C., Zhang S., Qi H., Zhang D., Li Y., et al. Novel advances in strategies and applications of artificial articular cartilage. Front Bioeng Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.987999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y., Chen J., Zhang Z., Lou K., Zhang Q., Wang S., et al. Current advances in the development of natural meniscus scaffolds: innovative approaches to decellularization and recellularization. Cell Tissue Res. 2017;370(1):41–52. doi: 10.1007/s00441-017-2605-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lehmann J., Nurnberger S., Narcisi R., Stok K.S., van der Eerden B.C.J., Koevoet W., et al. Recellularization of auricular cartilage via elastase-generated channels. Biofabrication. 2019;11(3) doi: 10.1088/1758-5090/ab1436. [DOI] [PubMed] [Google Scholar]
- 36.Barthold J.E., Martin B.M., Sridhar S.L., Vernerey F., Schneider S.E., Wacquez A., et al. Recellularization and integration of dense extracellular matrix by percolation of tissue microparticles. Adv Funct Mater. 2021;31(35) doi: 10.1002/adfm.202103355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia C., Mei S., Gu C., Zheng L., Fang C., Shi Y., et al. Decellularized cartilage as a prospective scaffold for cartilage repair. Mater Sci Eng C Mater Biol Appl. 2019;101:588–595. doi: 10.1016/j.msec.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Aoki F.G., Varma R., Marin-Araujo A.E., Lee H., Soleas J.P., Li A.H., et al. De-epithelialization of porcine tracheal allografts as an approach for tracheal tissue engineering. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-48450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qu M., Jiang X., Zhou X., Wang C., Wu Q., Ren L., et al. Stimuli-Responsive delivery of growth factors for tissue engineering. Adv Healthcare Mater. 2020;9(7) doi: 10.1002/adhm.201901714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Z., Cao F., Li H., He S., Zhao T., Deng H., et al. Microenvironmentally optimized 3D-printed TGFbeta-functionalized scaffolds facilitate endogenous cartilage regeneration in sheep. Acta Biomater. 2022;150:181–198. doi: 10.1016/j.actbio.2022.07.029. [DOI] [PubMed] [Google Scholar]
- 41.Lu S., Lam J., Trachtenberg J.E., Lee E.J., Seyednejad H., van den Beucken J., et al. Dual growth factor delivery from bilayered, biodegradable hydrogel composites for spatially-guided osteochondral tissue repair. Biomaterials. 2014;35(31):8829–8839. doi: 10.1016/j.biomaterials.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meyerrose T., Olson S., Pontow S., Kalomoiris S., Jung Y., Annett G., et al. Mesenchymal stem cells for the sustained in vivo delivery of bioactive factors. Adv Drug Deliv Rev. 2010;62(12):1167–1174. doi: 10.1016/j.addr.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Capito R.M., Spector M. Collagen scaffolds for nonviral IGF-1 gene delivery in articular cartilage tissue engineering. Gene Ther. 2007;14(9):721–732. doi: 10.1038/sj.gt.3302918. [DOI] [PubMed] [Google Scholar]
- 44.Yi Q., Xu Z., Thakur A., Zhang K., Liang Q., Liu Y., et al. Current understanding of plant-derived exosome-like nanoparticles in regulating the inflammatory response and immune system microenvironment. Pharmacol Res. 2023;190 doi: 10.1016/j.phrs.2023.106733. [DOI] [PubMed] [Google Scholar]
- 45.Liu S., Yu J.M., Gan Y.C., Qiu X.Z., Gao Z.C., Wang H., et al. Biomimetic natural biomaterials for tissue engineering and regenerative medicine: new biosynthesis methods, recent advances, and emerging applications. Mil Med Res. 2023;10(1):16. doi: 10.1186/s40779-023-00448-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matai I., Kaur G., Seyedsalehi A., McClinton A., Laurencin C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials. 2020;226 doi: 10.1016/j.biomaterials.2019.119536. [DOI] [PubMed] [Google Scholar]
- 47.Xue S., Ruan G., Li J., Madry H., Zhang C., Ding C. Bio-responsive and multi-modality imaging nanomedicine for osteoarthritis theranostics. Biomater Sci. 2023;11(15):5095–5107. doi: 10.1039/d3bm00370a. [DOI] [PubMed] [Google Scholar]
- 48.Li J., Cao F., Wu B., Yang J., Xu W., Wang W., et al. Immobilization of bioactive vascular endothelial growth factor onto Ca-deficient hydroxyapatite-coated Mg by covalent bonding using polydopamine. J Orthop Translat. 2021;30:82–92. doi: 10.1016/j.jot.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee K.I., Olmer M., Baek J., D'Lima D.D., Lotz M.K. Platelet-derived growth factor-coated decellularized meniscus scaffold for integrative healing of meniscus tears. Acta Biomater. 2018;76:126–134. doi: 10.1016/j.actbio.2018.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen W., Xu Y., Li H., Dai Y., Zhou G., Zhou Z., et al. Tanshinone IIA delivery silk fibroin scaffolds significantly enhance articular cartilage defect repairing via promoting cartilage regeneration. ACS Appl Mater Interfaces. 2020;12(19):21470–21480. doi: 10.1021/acsami.0c03822. [DOI] [PubMed] [Google Scholar]
- 51.Bicho D., Ajami S., Liu C., Reis R.L., Oliveira J.M. Peptide-biofunctionalization of biomaterials for osteochondral tissue regeneration in early stage osteoarthritis: challenges and opportunities. J Mater Chem B. 2019;7(7):1027–1044. doi: 10.1039/c8tb03173h. [DOI] [PubMed] [Google Scholar]
- 52.Song H., Park K.H. Regulation and function of SOX9 during cartilage development and regeneration. Semin Cancer Biol. 2020;67(Pt 1):12–23. doi: 10.1016/j.semcancer.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 53.Yang S., Qian Z., Liu D., Wen N., Xu J., Guo X. Integration of C-type natriuretic peptide gene-modified bone marrow mesenchymal stem cells with chitosan/silk fibroin scaffolds as a promising strategy for articular cartilage regeneration. Cell Tissue Bank. 2019;20(2):209–220. doi: 10.1007/s10561-019-09760-z. [DOI] [PubMed] [Google Scholar]
- 54.Ghafouri-Fard S., Poulet C., Malaise M., Abak A., Mahmud Hussen B., Taheriazam A., et al. The emerging role of non-coding RNAs in osteoarthritis. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.773171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen Y., Chen W., Ren Y., Li S., Liu M., Xing J., et al. Lipid nanoparticle-encapsulated VEGFa siRNA facilitates cartilage formation by suppressing angiogenesis. Int J Biol Macromol. 2022;221:1313–1324. doi: 10.1016/j.ijbiomac.2022.09.065. [DOI] [PubMed] [Google Scholar]
- 56.De Bari C., Roelofs A.J. Stem cell-based therapeutic strategies for cartilage defects and osteoarthritis. Curr Opin Pharmacol. 2018;40:74–80. doi: 10.1016/j.coph.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 57.Lam A.T.L., Reuveny S., Oh S.K. Human mesenchymal stem cell therapy for cartilage repair: review on isolation, expansion, and constructs. Stem Cell Res. 2020;44 doi: 10.1016/j.scr.2020.101738. [DOI] [PubMed] [Google Scholar]
- 58.Krajewska-Wlodarczyk M., Owczarczyk-Saczonek A., Placek W., Osowski A., Wojtkiewicz J. Articular cartilage aging-potential regenerative capacities of cell manipulation and stem cell therapy. Int J Mol Sci. 2018;19(2) doi: 10.3390/ijms19020623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cui L., Yang Z., Hong J., Zhu Z., Wang Z., Liu Z., et al. Injectable and degradable POSS-Polyphosphate-Polysaccharide hybrid hydrogel scaffold for cartilage regeneration. ACS Appl Mater Interfaces. 2023;15(17):20625–20637. doi: 10.1021/acsami.2c22947. [DOI] [PubMed] [Google Scholar]
- 60.Najafi R., Chahsetareh H., Pezeshki-Modaress M., Aleemardani M., Simorgh S., Davachi S.M., et al. Alginate sulfate/ECM composite hydrogel containing electrospun nanofiber with encapsulated human adipose-derived stem cells for cartilage tissue engineering. Int J Biol Macromol. 2023;238 doi: 10.1016/j.ijbiomac.2023.124098. [DOI] [PubMed] [Google Scholar]
- 61.Chen L., Yu C., Xiong Y., Chen K., Liu P., Panayi A.C., et al. Multifunctional hydrogel enhances bone regeneration through sustained release of Stromal Cell-Derived Factor-1alpha and exosomes. Bioact Mater. 2023;25:460–471. doi: 10.1016/j.bioactmat.2022.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen A., Chen Y., Rong X., You X., Wu D., Zhou X., et al. The application of exosomes in the early diagnosis and treatment of osteoarthritis. Front Pharmacol. 2023;14 doi: 10.3389/fphar.2023.1154135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tevlin R., desJardins-Park H., Huber J., DiIorio S.E., Longaker M.T., Wan D.C. Musculoskeletal tissue engineering: adipose derived stromal cell implementation for the treatment of osteoarthritis. Biomaterials. 2022;286 doi: 10.1016/j.biomaterials.2022.121544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang F.X., Liu P., Ding W., Meng Q.B., Su D.H., Zhang Q.C., et al. Injectable Mussel-Inspired highly adhesive hydrogel with exosomes for endogenous cell recruitment and cartilage defect regeneration. Biomaterials. 2021;278 doi: 10.1016/j.biomaterials.2021.121169. [DOI] [PubMed] [Google Scholar]
- 65.Liu Y., Dzidotor G., Le T.T., Vinikoor T., Morgan K., Curry E.J., et al. Exercise-induced piezoelectric stimulation for cartilage regeneration in rabbits. Sci Transl Med. 2022;14(627) doi: 10.1126/scitranslmed.abi7282. [DOI] [PubMed] [Google Scholar]
- 66.Makris E.A., Gomoll A.H., Malizos K.N., Hu J.C., Athanasiou K.A. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 2015;11(1):21–34. doi: 10.1038/nrrheum.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen R., Pye J.S., Li J., Little C.B., Li J.J. Multiphasic scaffolds for the repair of osteochondral defects: outcomes of preclinical studies. Bioact Mater. 2023;27:505–545. doi: 10.1016/j.bioactmat.2023.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramzan F., Salim A., Khan I. Osteochondral tissue engineering dilemma: scaffolding trends in regenerative medicine. Stem Cell Rev Rep. 2023;19(6):1615–1634. doi: 10.1007/s12015-023-10545-x. [DOI] [PubMed] [Google Scholar]
- 69.Jones C.L., Penney B.T., Theodossiou S.K. Engineering cell-ECM-material interactions for musculoskeletal regeneration. Bioengineering (Basel) 2023;10(4) doi: 10.3390/bioengineering10040453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bei H.P., Hung P.M., Yeung H.L., Wang S., Zhao X. Bone-a-Petite: engineering exosomes towards bone, osteochondral, and cartilage repair. Small. 2021;17(50) doi: 10.1002/smll.202101741. [DOI] [PubMed] [Google Scholar]
- 71.Lesage C., Lafont M., Guihard P., Weiss P., Guicheux J., Delplace V. Material-assisted strategies for osteochondral defect repair. Adv Sci. 2022;9(16) doi: 10.1002/advs.202200050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Badhe R.V., Chatterjee A., Bijukumar D., Mathew M.T. Current advancements in bio-ink technology for cartilage and bone tissue engineering. Bone. 2023;171 doi: 10.1016/j.bone.2023.116746. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.