Abstract
Inflammatory myofibroblastic tumor (IMT) stands as a rare neoplasm, initially documented by Bahadori and Liebow in 1973; however, its biological behavior and underlying pathogenesis continue to elude comprehensive understanding. Throughout the years, this tumor has been designated by various alternative names, including pseudosarcomatoid myofibroblastoma, fibromyxoid transformation, and plasma cell granuloma among others. In 2002, the World Health Organization (WHO) officially classified it as a soft tissue tumor and designated it as IMT. While IMT primarily manifests in the lungs, the common clinical symptoms encompass anemia, low-grade fever, limb weakness, and chest pain. The mesentery, omentum, and retroperitoneum are subsequent sites of occurrence with intracranial involvement being exceedingly rare. Due to the absence of specific clinical symptoms and characteristic radiographic features, diagnosing intracranial inflammatory myofibroblastic tumor (IIMT) remains challenging. Successful instances of pharmacological treatment for IIMT indicate that surgery may not be the sole therapeutic recourse, thus underscoring the imperative of an accurate diagnosis and apt treatment selection to improve patient outcomes.
Keywords: inflammatory myofibroblastic tumor, MRI, acoustic neuroma, surgical resection, headache
1. Introduction
IMT is a rare junctional/low-grade tumor with the potential for recurrence and progression [1]; it is mainly composed of myofibroblast spindle cells with inflammatory cell infiltration, including plasma cells, lymphocytes, and eosinophils [2]. In 2002, the WHO officially defined it as a soft tissue tumor and named it IMT [3]. The occurrence of IMT may be related to numerous factors, such as Epstein–Barr virus infection [4], surgery, trauma, etc. In addition, rearrangement and overexpression of the anaplastic lymphoma kinase (ALK) gene on chromosome 2p23 are also associated with IMT [5]. In 2000, Lawrence et al. found that the TPM3–ALK and TPM4–ALK fusion genes were related to IMT [6]. Since then, numerous ALK fusion genes have been reported, such as ATIC–ALK, CLTC–ALK, CARS–ALK, RANBP2–ALK, RRBP1–ALK, etc. [5,7,8]. In IMT, approximately 50% of ALK genes are rearranged and overexpressed; therefore, ALK positivity is helpful to diagnose IMT, but its absence does not exclude the diagnosis of IMT, particularly in adults [9,10]. The characteristic histological patterns include the fasciitis-like, compact spindle cell and hypocellular fibrous patterns [7,9]. While the most common site of occurrence is the lungs, extrapulmonary IMT tends to exhibit a more aggressive behavior and a higher susceptibility to recurrence [11]. The first reported case of IIMT was presented by SG West et al. in 1980 [12]. IIMT is extremely rare, and its clinical presentation often lacks a correlation with its histological type. Patients typically present with non-specific symptoms, such as headaches and seizures among others [13]. The preoperative diagnosis of IIMT poses challenges. Previous radiological studies have primarily depicted IIMT as a solid mass; however, Wang [14] and Park [15], among others, have also identified instances of cystic IIMT. This substantiates the notion that there is no distinct morphological component characteristic of IIMT evident in radiographic imaging.
The current body of literature concerning IIMT primarily comprises case reports, thus lacking a comprehensive overview. This article presents the first instance of hemorrhagic IIMT treated at the First Affiliated Hospital of Soochow University, which was initially misdiagnosed as an acoustic neuroma. Additionally, we conducted a comprehensive review of the relevant literature on IIMT from 1980 to 2022 using the PubMed database, aiming to enhance our understanding of this rare neoplasm.
2. Case Presentation
On 27 July 2022, a 55-year-old male presented at the Second People’s Hospital in Changshu, Jiangsu Province, China with unexplained symptoms of dizziness, unsteady walking, choking on water, and hoarseness. The attending doctor initially diagnosed an acoustic neuroma in the right cerebellopontine angle. Subsequently, the patient sought further treatment at our hospital. The patient had a history of hypertension for over 20 years but had stopped taking medication on his own for three years, perceiving stable blood pressure. Additionally, he had a history of radiotherapy for nasopharyngeal cancer 20 years ago and had been regularly followed up after discharge. Routine laboratory tests showed increased neutrophils, leukocytes, and C-reactive protein with other values within normal limits. The chest computed tomography (CT) examination revealed no abnormalities.
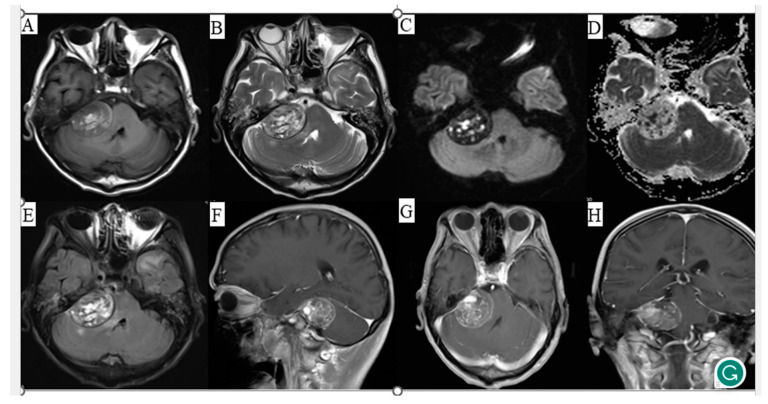
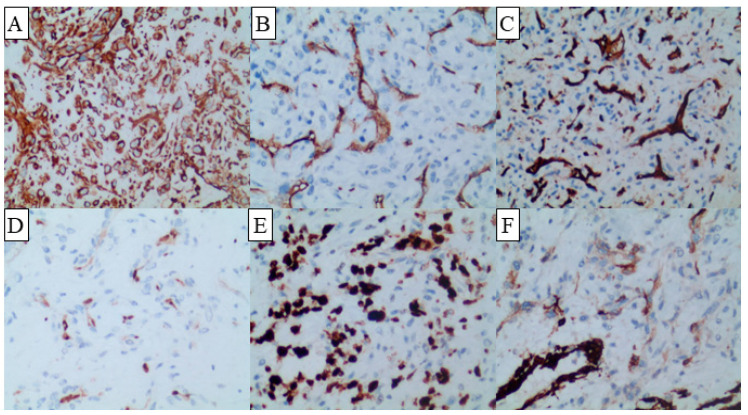
Cranial magnetic resonance imaging (MRI) demonstrated a circular mixed T1-weighted imaging (T1WI) and T2-weighted imaging (T2WI) signal shadow in the right cerebellopontine angle with a lesion size of approximately 3.9 × 3.2 × 3.0 cm, mixed diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) signals, and heterogeneous enhancement. The compression of the right cerebellar hemisphere and the four ventricles resulted in their displacement towards the left (Figure 1). The neuroradiologist at our hospital also diagnosed it as an acoustic neuroma. The patient underwent tumor resection, and histopathological analysis revealed a lesion comprised of fibrous collagen tissue with hemorrhagic infarcts and lamellar capillary hyperplasia. Within the lesion, there were perivascular spindle cells and epithelioid proliferation, exhibiting cellular heterogeneity and easily visible nuclear pleomorphism. Immunohistochemical staining demonstrated negativity for glial fibrillary acidic protein (GFAP), S100 protein, Oligodendrocyte transcription factor 2 (Olig-2), CD56, SOX10, and epithelial membrane antigen (EMA). Additionally, the lesion was negative for estrogen receptor (ER), progesterone receptor (PR), somatostatin receptor 2 (SSTR2), cytokeratin (CK), desmin, and CD68. The lesion displayed positivity for vimentin and exhibited scattered weak positivity for smooth muscle actin (SMA) in the vascular regions (Figure 2). Furthermore, immunostaining revealed CD34 and CD31 positivity in the vascular regions as well as erythroblast transformation specific related gene (ERG) and Ki-67 (approximately 40% in the hotspot areas) positivity (Figure 2). Based on these findings and the WHO classification guidelines (Table 1, the postoperative pathology confirmed an intracranial inflammatory myofibroblastic tumor.
Figure 1.
Magnetic resonance imaging (MRI) of the patient’s head with corresponding image sequences. (A) T1-weighted imaging, (B) T2-weighted imaging, (C) Diffusion-weighted imaging, (D) Apparent diffusion coefficient, (E) Fluid attenuated inversion recovery, (F) Sagittal contrast-enhanced scan, (G) Axial contrast-enhanced scan, (H) Coronal contrast-enhanced scan.
Figure 2.
Immunohistochemical staining of the lesion demonstrated positive reactivity for vimentin (A) throughout and revealed scattered weak positivity for SMA (B) in the vascular regions. Additionally, the vascular regions exhibited positive staining for CD31 (C), ERG (D), Ki-67 (E), and CD34 (F). The original magnification is 20X.
Table 1.
WHO classification of fibroblastic/myofibroblastic and some representative immunomarkers.
| Type of Tumor | Representative Immunomarkers |
|---|---|
| Nodular fasciitis | CD34 (+), SMA (+), Desmin (−), S100 (−), ALK-1 (−), Ki-67(+) |
| Proliferative fasciitis | SMA (+), Desmin (−), CD34 (+), S100 (−), ALK-1 (−), Ki-67 (+) |
| Proliferative myositis | SMA (+), CD34 (+), Desmin (−), S100 (−), ALK-1 (−), Ki-67 (+) |
| Myositis ossificans | SMA (+), Desmin (−), CD34 (−), S100(−), ALK-1 (−), Ki-67 (−) |
| Ischaemic fasciitis | SMA (+), Desmin (−), CD34 (+), S100(−), ALK-1 (−), Ki-67 (+) |
| Elastofibroma | SMA (+), Desmin (−), CD34 (−), S100 (−), ALK-1 (−), Ki-67 (−) |
| Fibrous hamartoma of infancy | SMA (+), Desmin (−), CD34 (+), S100(−), ALK-1 (−), Ki-67 (+) |
| Myofibroma/Myofibromatosis | SMA (+), Desmin (−), CD34 (−), S100 (−), ALK-1 (−), Ki-67 (+) |
| Fibromatosis colli | SMA (+), Desmin (−), CD34 (−), S100 (−), ALK-1 (−), Ki-67 (−) |
| Juvenile hyaline fibromatosis | Vimentin (+), CD34 (−), CD68 (−), SMA (−),S 100(−), Factor XIIIa (−) |
| Inclusion body fibromatosis | Vimentin (+), CD34 (−), CD68 (−), SMA (−), S100 (−), Desmin (−) |
| Fibroma of tendon sheath | Vimentin (+), CD34 (−), Desmin (−), SMA (−), S100 (−), CD68 (−) |
| Desmoplastic fibroblastoma | Vimentin (+), CD34 (−), Desmin (−), SMA (−), S100(−), CD68 (−) |
| Mammary-type myofibroblastoma | Vimentin (+), CD34 (−), Desmin (−), SMA (+), S100 (−), CD68 (−) |
| Calcifying aponeurotic fibroma | Vimentin (+), CD34 (−), Desmin (−), SMA (−), S100 (−), CD68 (−) |
| Angiomyofibroblastoma | Vimentin (+), CD34 (−), Desmin (−), SMA (+), S100 (−), CD68 (−) |
| Cellular angiofibroma | Vimentin (+), CD34 (+), Desmin (−), SMA (−), S100 (−), CD68 (−) |
| Nuchal-type fibroma | Vimentin (+), CD34 (−), Desmin (−), SMA (−), S100 (−), CD68 (−) |
| Gardner fibroma | Vimentin (+), CD34 (−), Desmin (−), SMA (−), S100 (−), CD68 (−) |
| Calcifying fibrous tumor | Vimentin (+), CD34 (−), Desmin (−), SMA (−), S100 (−), CD68 (−) |
| Giant cell angiofibroma | Vimentin (+), CD34 (−), Desmin (−), SMA (+), S100 (−), CD68 (−) |
| Superficial fibromatoses (palmar/plantar) | SMA (+), CD34 (−), Desmin (−), S100 (−), Beta-catenin(CTNNB1) (+) |
| Desmoid-type fibromatoses | SMA (+), CD34 (−), Desmin (−), S100 (−), Beta-catenin(CTNNB1) (+) |
| Lipofibromatosis | CD34 (+), SMA (+), Desmin (−), S100 (−), Beta-catenin(CTNNB1) (−) |
| Solitary fibrous tumor | CD34 (+), CD99 (+), Bcl-2 (+), CD31 (−), S100 (−), Desmin (−) |
| Inflammatory myofibroblastic tumor | ALK [partly(+)], SMA (+), CD34 (+), Desmin (−), CD117 (−), S100 (−) |
| Low grade myofibroblastic sarcoma | SMA (+), Desmin (−), CD34 (−), CD117 (−), ALK (−), S100 (−) |
| Myxoinflammatory | CD68 (+), CD163 (+), SMA (+), Desmin (−), CD34 (−), S100 (−) |
| Infantile fibrosarcoma | SMA (+), Desmin (−), CD34 (−), CD99 (−), ALK (−), S100 (−) |
| Adult fibrosarcoma | SMA (+), CD34 (−), Desmin (−), S100 (−), EMA (−), CD99 (−) |
| Myxofibrosarcoma | CD34 (−), SMA (−), Desmin (−), S100 (−), EMA (−), CD99 (−) |
| Low grade fibromyxoid sarcoma | CD34 (−), SMA (−), Desmin (−), S100 (−), EMA (−), MUC4 (+) |
| Sclerosing epithelioid fibrasarcoma | CD34 (−), SMA (−), Desmin (−), S100 (−), EMA (−), INI1(SMARCB1):(+) |
Ki-67: Ki-67 Antigen; Factor XIIIa: Factor XIIIa (F13A1); Beta-catenin (CTNNB1): Beta-Catenin; INI1 (SMARCB1): SWI/SNF-related matrix-associated actin dependent regulator of chromatin subfamily B member 1; Bcl-2: B-Cell Lymphoma 2.
Approximately one year after hospital discharge, we conducted a follow-up via phone call, during which the patient reported no notable discomfort or concerns.
3. Literature Review
This misdiagnosed case prompted us to conduct an in-depth investigation into IIMT. Due to the historical use of various names for this tumor, we conducted a comprehensive search of the PubMed database using the following keywords: “inflammatory myofibroblastic tumor,” “intracranial plasma cell granuloma,” “inflammatory pseudotumor,” “cellular inflammatory pseudotumor,” “fibrous yellow tumor,” “yellow tumor pseudotumor,” “pseudosarcomatous myofibroblast proliferation,” and “inflammatory myofibrohistiocytic proliferation”. We selected articles encompassing case reports, reviews, and other relevant studies for analysis. The literature was carefully reviewed to extract the pertinent information, such as the tumor’s location of occurrence, clinical manifestations, treatment modalities, and more. The results are shown in Table 2.
After excluding literature with unavailable content, a total of 55 cases were included in the study until October 2022. The age range of patients varied from 6 to 82 years with 23 females (41.8%) and 32 males (58.2%). While IMT primarily occurs in the lung and respiratory tract, it has also been reported in various other sites, such as the breast, esophagus, intestine, kidney, liver, lymph nodes, retroperitoneum, oral cavity, skin, stomach, thyroid, etc. However, the occurrence of IMT within the intracranial region remains exceptionally rare. From Table 2, we observed that IIMT can arise in almost all intracranial locations with the frontal (23.6%) and temporal lobes (21.8%) being the main sites. The clinical symptoms commonly included headache (56.4%) and seizures (18.2%) with headache being the predominant complaint. A few cases presented with metastasis (three cases) and recurrence (ten cases) with the earliest recurrence noted six months after treatment, and the latest recurrence occurring in the 11th year. Regarding the treatment of IIMT, various strategies were employed, including surgery, glucocorticoids, radiotherapy, chemotherapy, immunosuppressants, 6-mercaptopurine, methotrexate, non-steroidal anti-inflammatory drugs (NSAIDs), thalidomide, and amphotericin. From Table 2, we observed that most cases exhibited iso or low signal intensity on T1WI and T2WI with the DWI signal usually being low. Moreover, most of the lesions showed significant enhancement (although some were not homogeneous). Table 2 also lists the initial diagnoses made by the clinicians and neuroradiologists before the pathological results were obtained, based only on the radiological features and clinical symptoms. Interestingly, meningioma (30.9%) ranked first among the preoperative misdiagnosed diseases, similar to what has been stated in other articles; in terms of radiological presentations, IIMT resembles meningiomas [16], including enhancing features and the typical meningeal tail sign. Among all the cases listed in Table 2, there was no instance in which IIMT could be conclusively diagnosed solely based on the radiological features. This observation further underscores the intricate nature of diagnosing IIMT.
4. Discussion
IMT represents a rare neoplasm with uncertain biological behavior and underlying pathogenesis. Despite the formal definition of IMT by the WHO in 2002, certain articles published after this date still lack a clear differentiation between IMT, inflammatory pseudotumor, and other histologically akin lesions, a point also noted by Ishihara, Denis, and Vidrine [11,17,18] et al. The majority of the IIMT showed unique MRI performance: iso or low signal on TIWI and T2WI, which may be attributed to the absence of free water and mobile protons [13]. Additionally, IIMT exhibited prominent enhancement and low signal intensity on DWI. These MRI findings were different from other common intracranial parenchymal tumors, which typically present as low-intensity lesions on T1WI and high-intensity lesions on T2WI. This distinctive feature of IIMT is noteworthy in the context of radiological differentiation. In our patient diagnosed with IIMT, the T1WI and T2WI signals predominantly appeared low; however, a small area of high signal intensity was observed within the lesion. The postoperative pathology confirmed the presence of minor hemorrhage, marking the first reported case of IIMT with hemorrhage. The lesion was situated at the right cerebellopontine angle and demonstrated relatively well-defined characteristics. Consequently, the preoperative assessment by the neuroradiologist considered a right cerebellopontine angle acoustic neuroma with hemorrhage. It is noteworthy that the tumor’s atypical T1WI and T2WI signals are distinct from the usual acoustic neuroma. Considering this unique imaging characteristic, the possibility of IIMT could be contemplated, especially if there had been previous IIMT-related studies. In addition to the radiological features, the two main clinical manifestations of headache and seizures might be informative for the diagnosis of IIMT, which has been mentioned in previous reports [13,19].
In the context of this study, the panel of immune markers, including CD34, CD31, ERG, Ki-67, SMA, Vimentin, and others, assumes a pivotal role in the elucidation of both the histological attributes and the molecular profile of IIMT. CD34 and CD31 recognized endothelial markers play a crucial role in the identification of vascular constituents within the lesion [20]. The presence of positive staining for these markers indicates the presence of blood vessels and provides insight into the vascular architecture within the tumor mass. ERG, functioning as an endothelial transcription factor, further contributes to a refined comprehension of the vascular structures and the evaluation of tumor vascularity [20]. Affirmative ERG staining provides insights into the differentiation and arrangement of endothelial cells within the tumor tissue. Ki-67, a representative marker of proliferation, quantifies the proliferative activity of tumor cells. Its expression level, especially within focal hotspot regions, furnishes valuable data regarding the tumor’s growth rate and potential aggressive behavior [21]. SMA, or smooth muscle actin, denotes the presence of smooth muscle cells or myofibroblasts [22]. The scattered yet discernible weak positivity observed in the vascular regions imparts insights into the cellular composition and accentuates the presence of the smooth muscle component within the tumor. Vimentin, serving as a notable mesenchymal marker, signifies the mesenchymal origin of the tumor cells, facilitating their differentiation from other cell types [23]. The collective contribution of these immune markers contributes substantively to the comprehensive characterization of IMT, facilitating the precise diagnosis and comprehension of their biological behavior, and furnishing potential insights relevant to their management and treatment strategies.
ALK gene expression is suggestive of the development and recurrence of IIMT [17]; chemotherapeutic agents targeting ALK gene inhibition have also been used for the treatment of IIMT with good results [11]. Although some clinicians suggest that surgery is the preferred treatment option for IIMT [24], glucocorticoids have been successful in the treatment of IIMT in some cases [25,26]. Hence, a clear preoperative diagnosis of IIMT would allow for the possibility of an initial steroid-based therapeutic approach, potentially alleviating patient discomfort and reducing postoperative complications, should it prove effective.
Table 2.
Information about inflammatory myofibroblastic tumor.
| Patient [Ref] | Location | Complaints | Meningeal Tail Sign | Metastasis | Treatment | Radiological Manifestations | Misdiagnosis | Recurrence |
|---|---|---|---|---|---|---|---|---|
| 17/M [12] | left posterior fossa | headache | NA | ➖ | S | obviously enhancing mass | meningioma | ➖ |
| 6/M [27] | bitemporal lobe | deafness, right hemiparesis, and bilateral cerebellar signs | ➖ | ➖ | S | CT: obviously enhancing mass | NA | no recurrence at 6 years |
| 14/F [28] | right posteromedial frontal lobe | headache | ➖ | ➖ | S+H+R | obviously enhancing mass | meningioma | recurrence at 6 months |
| 48/F [29] | hypothalamus | drowsiness, hyperthermia, vomiting, and headache | ➖ | ➖ | NA | NA | NA | ➖ |
| 19/F [29] | hypothalamus | visual loss, headaches, and drowsiness | ➖ | ➖ | S+R | NA | NA | NA |
| 16/M [30] | right frontoparietal convexity | progressive weakness in the left leg | NA | ➖ | S | CT: iso-density mass | meningioma | ➖ |
| 77/F [31] | left frontal region and clival | dementia, urinary incontinence, and anorexia | ➖ | NA | H | low SI on both T2WI and T1WI, homogeneous enhancement | chronic subdural hematoma | ➖ |
| 40/M [32] | left trigeminal nerve and left cavernous sinus | decrease in visual acuity or loss of visual field | ➖ | ➖ | S+H | T1WI: iso-SI, obviously enhancing | NA | ➖ |
| 57/M [33] | on the falx cerebri in the frontal area | headache | ➖ | ➖ | S | NA | NA | NA |
| 56/M [34] | pituitary stalk | frontal cephalalgias | NA | ➖ | S | T1WI: iso-SI, obviously enhancing | NA | ➖ |
| 30/M [34] | left cavernous sinus and tentorium cerebelli | parieto-occipital cephalalgias | NA | ➖ | S | T1WI: low-SI | NA | ➖ |
| 11/M [34] | vermis cerebelli | occipital cephalalgias | NA | ➖ | S | T1WI: low-SI; T2WI: low-SI at the edge of the lesion and iso-SI in the center | NA | ➖ |
| 40/M [34] | right cavernous sinus | right fronto-orbicular cephalalgias | NA | ➖ | S | CT: obviously enhancing; TIWI, T2WI: high-SI |
meningioma | recurrence at 2 years |
| 11/M [35] | left frontal lobe | mild headache and nausea | ➖ | ➖ | S | T1WI: slightly high-SI T2WI: low-SI, heterogeneous enhancement |
NA | ➖ |
| 60/F [36] | near the anterior tip of the temporal lobe | headache, grand mal seizure, and postictal confusion | ➕ | ➖ | S+R | T1WI, T2WI: low-SI, obviously enhancing | NA | ➖ |
| 57/F [37] | right cerebellopontine angle | right-sided ptosis and diplopia | NA | ➖ | S+R | NA | NA | NA |
| 17/F [1] | left frontal | left frontal headache | NA | ➖ | S | NA | NA | recurrence at 2 years |
| 8/M [1] | left temporal | seizure | NA | ➖ | S | NA | NA | no recurrence at 5 years |
| 15/M [1] | left occipital | right-sided epileptic seizure | NA | intracranial MT | S+R | obviously enhancing mass | NA | recurrence at 6 months |
| 18/F [38] | right temporal region | headache with occasional vomiting and blurring of vision | ➖ | ➖ | S | CT: obviously enhancing mass | meningioma | ➖ |
| 23/M [39] | right parieto-occipital | seizures | ➕ | ➖ | S+H | T1WI: low-SI T2WI: high-SI slightly enhancing |
meningioma | no recurrence at 3 years |
| 13/M [19] | right frontal lobe | seizures | ➖ | ➖ | S | T2WI: hypo-intense Obviously enhancing |
NA | no recurrence at 6 months |
| 62/M [40] | right fronto-parietooccipital and falx | focal motor seizures and right-sided tinnitus | ➕ | ➖ | S+R | T1WI: iso-SI T2WI: low-SI obviously enhancing |
meningioma | ➖ |
| 6/M [41] | right parietal region | seizure | ➖ | ➖ | S | obviously enhancing | NA | ➖ |
| 41/M [42] | right occipital lobe | epileptic seizure | ➖ | intracranial MT | S | obviously enhancing | High grade glioma, brain metastasis tumor | recurrence at 11 years |
| 22/M [43] | under surface of left tentorium | headache | ➕ | ➖ | S | obviously enhancing | meningioma | no recurrence at 19 months |
| 64/F [44] | beside the anterior parasagittal region | headache | NA | ➖ | S | obviously enhancing | meningioma | no recurrence at 3 years |
| 70/M [45] | cranial base, frontal region, floor of the third ventricle | progressive visual disturbance | ➖ | NA | H+R | obviously enhancing | pituitary tumor, chordoma, plasmacytoma |
NA |
| 36/F [42] | left cerebellopontine angle | left-sided headache, tinnitus, and hearing loss | NA | MT to cervical | S+R | obviously enhancing | meningioma | recurrence at 1.5 years |
| 52/M [46] | left cerebellopontine angle | decreased sensation on the left side of the face, hearing loss, headache, and vomiting | ➖ | ➖ | S | T1WI: iso-SI, T2WI: low-SI heterogeneous enhancement |
trigeminal neurinoma, meningioma | no recurrence at 6 months |
| 30/F [47] | left temporo-parietal extra parenchymal | worsening headache and memory disturbance | NA | ➖ | S | CT: iso-density mass obviously enhancing |
subacute subdural hematoma | ➖ |
| 48/F [47] | left temporal lobe | headache | NA | ➖ | S | T1: low-SI, T2: high-SI obviously enhancing |
NA | ➖ |
| 14/F [48] | cavernous sinus, right middle cranial fossa, pterygopalatine, and infratemporal | headache | ➖ | ➖ | H+methotrexate +6-mercaptopurine | NA | NA | recurrence at 18 months |
| 63/M [49] | right frontal lobe | progressive left hemiparesisi | ➖ | ➖ | S | MRI: the solid part of the mass was significantly enhanced | NA | ➖ |
| 18/F [50] | left frontoparietal | generalized seizure | ➖ | ➖ | S | CT: calcifications and extensive ossification T1: low-SI |
NA | ➖ |
| 58/F [51] | left fronto-temporal | headache | ➖ | ➖ | S+H | obviously enhancing mass | NA | no recurrence at 18 months |
| 47/M [52] | right cerebellum-pontine angle | reduced visual acuity, hearing loss, difficulty in walking, and urinary retention | NA | NA | H+R | T1WI: iso-SI, T2WI: low-SI | NA | NA |
| 26/F [53] | left frontotemporal region | severe headache, left eye discomfort with diplopia, left ear pain | NA | NA | high-dose dexamethasone and thalidomide | MRI: extensive enhancing, dural thickening over left frontotemporal lobes | NA | NA |
| 52/F [53] | right lateral ventricle | slipped and fell with head injury | NA | NA | S | NA | NA | NA |
| 45/M [53] | right frontal region | progressive left-sided weakness | NA | NA | S | CT: right frontal dural based tumor with peritumoral edema | NA | NA |
| 51/M [26] | right cerebellopontine angle | vertigo, diplopia, headache and fibrillationa of the tongue | NA | ➖ | H | obviously enhancing mass | NA | recurrence at 7 years |
| 56/M [54] | left basal ganglia | headaches and right-sided weakness | ➖ | ➖ | H+biopsy | TIWI: low-signal, heterogeneous enhancement |
NA | ➖ |
| 60/M [55] | right cerebral hemisphere | gait disturbance and ataxia | ➖ | ➖ | S | T1WI, T2WI: iso-SI, obviously enhancing |
NA | ➖ |
| 82/F [56] | right temporal region | headache and memory decrease | ➕ | ➖ | S | T1WI: slightly high-SI homogeneous enhancement |
meningioma | no recurrence at 13 months |
| 38/M [57] | right mastoid | blurred vision and headache | ➖ | ➖ | S+H | MRI: obviously enhancing CT: bone erosion around the tumor MRV: right sigmoid sinus occlusion |
NA | ➖ |
| 48/F [15] | right temporal region | depression, paranoid personality, and memory impairment | ➖ | ➖ | S | T2WI: iso-SI, DWI: not limited, obviously enhancing | pleomorphic xanthoastrocytoma, cystic meningioma, cystic glioma | ➖ |
| 20/F [58] | bilateral temporal regions | headache | NA | ➖ | S | T1WI: low-SI T2WI: mixed-SI CT: iso-density mass heterogeneous enhancement |
NA | recurrence at 7 months |
| 10/M [2] | left transverse-sigmoid junction | mastoid tenderness and headache | ➕ | ➖ | S | CT: obviously enhancing | meningioma | ➖ |
| 15/M [59] | left parietal region | headache, vomiting, and lethargy | ➖ | ➖ | S | T1WI: iso-SI T2WI: low-SI obviously enhancing |
NA | NA |
| 46/F [14] | right temporal lobe and right cerebellar hemisphere | headache, unstable walking | NA | NA | S+H | T1WI: iso-SI T2WI: low-SI MRV: right transverse sinus and sigmoid sinus occlusion |
malignant meningioma | recurrence at 2.5 years |
| 21/F [5] | right frontal lobe | bilateral blurred vision | ➖ | ➖ | S | T1WI, T2WI: low-SI obviously enhancing |
meningioma | ➖ |
| 54/M [14] | right frontal lobe | headache | ➕ | NA | S | T1WI: iso-SI T2WI: low-SI obviously enhancing |
NA | ➖ |
| 27/M [16] | right frontal parietal region | seizures | ➕ | NA | S | obviously enhancing | meningioma | NA |
| 80/F [60] | left choroidal fissure between the amygdala and cerebral peduncle | headache, dizziness, confusion, and gait instability | ➖ | ➖ | S | obviously enhancing | aneurysm | ➖ |
NA = non-available; S = surgery; H = hormones; R = radiotherapy; NSAIDs = non-steroidal anti-inflammatory drugs; CT = computed tomography; MRI = magnetic resonance imaging; T1WI = T1-weighted imaging; T2 = T2-weighted imaging; SI = signal intensity; MRV = magnetic resonance venography; DWI = diffusion-weighted imaging. ➕ = positive; ➖ = negative.
5. Conclusions
This study presents the inaugural case of IIMT with internal hemorrhage and undertakes an all-encompassing review of pertinent literature accessible in the PubMed database. While the direct diagnosis of IIMT presents challenges, considering IIMT as a plausible differential diagnosis emerges as a feasible avenue. This approach assumes substantial significance in steering treatment strategies effectively.
Author Contributions
Y.L. designed the study. Z.L., R.H. and W.P. collected the literature. L.Z. drafted the manuscript. Z.Y. collected the pathological sections. All authors were involved in major revisions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of First Hospital of Soochow University (approval code 2023-291, approved date: 7 July 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All of the data in this study can be found in the PubMed database.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was supported by the program for Gusu Medical Talent of Suzhou City (grant no. GSWS2020009); the Translational Research Grant of NCRCH (grant no. 2020WSB06); and the Youth Program of Suzhou Medical Association (grant no. 2022YX-Q03).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Häusler M., Schaade L., Ramaekers V.T., Doenges M., Heimann G., Sellhaus B. Inflammatory Pseudotumors of the Central Nervous System: Report of 3 Cases and a Literature Review. Hum. Pathol. 2003;34:253–262. doi: 10.1053/hupa.2003.35. [DOI] [PubMed] [Google Scholar]
- 2.Singhal N., Agarwal V., Chawla A., Tangri R. Central Nervous System Inflammatory Myofibroblastic Tumor Masquerading as Chronic Suppurative Otitis Media. J. Pediatr. Neurosci. 2017;12:188–191. doi: 10.4103/jpn.JPN_95_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO . Pathology and Genetics of Tumours of Soft Tissue and Bone. IARC; Lyon, France: 2002. [Google Scholar]
- 4.Fukunaga A., Yoshida K., Otani M., Ogawa Y., Horiguchi T., Ishihara M., Toya S., Kawase T. Plasma Cell Granuloma Extending from the Extracranial to the Intracranial Space Associated with Epstein-Barr Virus Infection Case Report. Neurol. Med. Chir. 1998;38:292–296. doi: 10.2176/nmc.38.292. [DOI] [PubMed] [Google Scholar]
- 5.Wang X., Chen Y., Wu X., Zhang H. Intracranial Inflammatory Myofibroblastic Tumor with Negative Expression of Anaplastic Lymphoma Kinase: A Case Report and Review of the Literature. World Neurosurg. 2019;125:117–122. doi: 10.1016/j.wneu.2019.01.155. [DOI] [PubMed] [Google Scholar]
- 6.Lawrence B., Perez-Atayde A., Hibbard M.K., Rubin B.P., Dal Cin P., Pinkus J.L., Pinkus G.S., Xiao S., Yi E.S., Fletcher C.D., et al. TPM3-ALK and TPM4-ALK Oncogenes in Inflammatory Myofibroblastic Tumors. Am. J. Pathol. 2000;157:377–384. doi: 10.1016/S0002-9440(10)64550-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Surabhi V.R., Chua S., Patel R.P., Takahashi N., Lalwani N., Prasad S.R. Inflammatory Myofibroblastic Tumors: Current Update. Radiol. Clin. N. Am. 2016;54:553–563. doi: 10.1016/j.rcl.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Lee J.-C., Li C.-F., Huang H.-Y., Zhu M.-J., Mariño-Enríquez A., Lee C.-T., Ou W.-B., Hornick J.L., Fletcher J.A. ALK Oncoproteins in Atypical Inflammatory Myofibroblastic Tumours: Novel RRBP1-ALK Fusions in Epithelioid Inflammatory Myofibroblastic Sarcoma. J. Pathol. 2017;241:316–323. doi: 10.1002/path.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gleason B.C., Hornick J.L. Inflammatory Myofibroblastic Tumours: Where Are We Now? J. Clin. Pathol. 2008;61:428–437. doi: 10.1136/jcp.2007.049387. [DOI] [PubMed] [Google Scholar]
- 10.Wang X., Krishnan C., Nguyen E.P., Meyer K.J., Oliveira J.L., Yang P., Yi E.S., Erickson-Johnson M.R., Yaszemski M.J., Maran A., et al. Fusion of Dynactin 1 to Anaplastic Lymphoma Kinase in Inflammatory Myofibroblastic Tumor. Hum. Pathol. 2012;43:2047–2052. doi: 10.1016/j.humpath.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Vidrine D.W., Berry J.F., Garbuzov A., Falcon C., Tubbs R.S., Bui C.J. DCTN1-ALK Gene Fusion in Inflammatory Myofibroblastic Tumor (IMT) of the CNS. Childs Nerv. Syst. 2021;37:2147–2151. doi: 10.1007/s00381-021-05219-3. [DOI] [PubMed] [Google Scholar]
- 12.West S.G., Pittman D.L., Coggin J.T. Intracranial Plasma Cell Granuloma. Cancer. 1980;46:330–335. doi: 10.1002/1097-0142(19800715)46:2<330::AID-CNCR2820460220>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 13.Kim J.-H., Chang K.-H., Na D.G., Park S.-H., Kim E., Han D.H., Kwon H.-M., Sohn C.-H., Yim Y.J. Imaging Features of Meningeal Inflammatory Myofibroblastic Tumor. AJNR Am. J. Neuroradiol. 2009;30:1261–1267. doi: 10.3174/ajnr.A1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang G., Shen H., Chu Z., Shen J., Zhang L. Intracranial Inflammatory Myofibroblastic Tumor: A Report of Two Cases. Br. J. Neurosurg. 2021;12:1–5. doi: 10.1080/02688697.2021.1879011. [DOI] [PubMed] [Google Scholar]
- 15.Park J.-H., Yoon W.-S., Chung D.-S. Unusual Radiologic Finding of Intracranial Inflammatory Myofibroblastic Tumor Presenting a Cyst with Mural Nodule. Brain. Tumor. Res. Treat. 2015;3:138–140. doi: 10.14791/btrt.2015.3.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phogat D., Datta S.G.S., Bajpai M., Tara S., Ganti S.K. Intracranial Inflammatory Myofibroblastic Tumor: A Review of 49 Cases. Autops. Case Rep. 2021;11:e2021254. doi: 10.4322/acr.2021.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denis D.J., Elayoubi K., Weil A.G., Berthelet F., Bojanowski M.W. Inflammatory Myofibroblastic Tumors of the Central Nervous System That Express Anaplastic Lymphoma Kinase Have a High Recurrence Rate. Surg. Neurol. Int. 2013;4:70. doi: 10.4103/2152-7806.112614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishihara M., Izumoto S., Iwatsuki K., Yoshimine T. Immunohistochemical Study of Multiple Inflammatory Pseudotumors with Both Brain and Spinal Cord Involvement—Case Report. Neurol. Med. Chir. 2010;50:246–250. doi: 10.2176/nmc.50.246. [DOI] [PubMed] [Google Scholar]
- 19.Greiner C., Rickert C.H., Möllmann F.T., Rieger B., Semik M., Heindel W., Wassmann H. Plasma Cell Granuloma Involving the Brain and the Lung. Acta. Neurochir. 2003;145:1127–1131. doi: 10.1007/s00701-003-0109-z. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan H.C., Edgar M.A., Cohen C., Kovach C.K., HooKim K., Reid M.D. The Utility of ERG, CD31 and CD34 in the Cytological Diagnosis of Angiosarcoma: An Analysis of 25 Cases. J. Clin. Pathol. 2015;68:44–50. doi: 10.1136/jclinpath-2014-202629. [DOI] [PubMed] [Google Scholar]
- 21.Li L.T., Jiang G., Chen Q., Zheng J.N. Ki67 Is a Promising Molecular Target in the Diagnosis of Cancer (Review) Mol. Med. Rep. 2015;11:1566–1572. doi: 10.3892/mmr.2014.2914. [DOI] [PubMed] [Google Scholar]
- 22.Tang D.D. The Dynamic Actin Cytoskeleton in Smooth Muscle. Adv. Pharmacol. 2018;81:1–38. doi: 10.1016/bs.apha.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Roles of Vimentin in Health and Disease—PubMed. [(accessed on 9 August 2023)]; Available online: https://pubmed.ncbi.nlm.nih.gov/35487686/
- 24.Güdük M., Yener U., Sav A., Pamir M.N. Intracranial Multifocal Plasma Cell Granuloma: A Case with Multiple Operations without Recurrence of Surgically Removed Lesions. Acta. Neurochir. 2016;158:721–723. doi: 10.1007/s00701-016-2724-5. [DOI] [PubMed] [Google Scholar]
- 25.Tresser N., Rolf C., Cohen M. Plasma Cell Granulomas of the Brain: Pediatric Case Presentation and Review of the Literature. Childs Nerv. Syst. 1996;12:52–57. doi: 10.1007/BF00573857. [DOI] [PubMed] [Google Scholar]
- 26.Carswell C., Chataway C. The Successful Long-Term Management of an Intracranial Inflammatory Myofibroblastic Tumor with Corticosteroids. Clin. Neurol. Neurosurg. 2012;114:77–79. doi: 10.1016/j.clineuro.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 27.Kamiryo T., Abiko S., Orita T., Aoki H., Watanabe Y., Hiraoka K. Bilateral Intracranial Fibrous Xanthoma. Surg. Neurol. 1988;29:27–31. doi: 10.1016/0090-3019(88)90119-X. [DOI] [PubMed] [Google Scholar]
- 28.Cannella D.M., Prezyna A.P., Kapp J.P. Primary Intracranial Plasma-Cell Granuloma. Case Report. J. Neurosurg. 1988;69:785–788. doi: 10.3171/jns.1988.69.5.0785. [DOI] [PubMed] [Google Scholar]
- 29.Ferrer I., Garcia Bach M., Aparicio M.A., Acebes J.J., Twose J., Isamat F. Plasma Cell Granuloma of the Hypothalamic Region. Acta. Neurochir. 1989;99:152–156. doi: 10.1007/BF01402325. [DOI] [PubMed] [Google Scholar]
- 30.Gangemi M., Maiuri F., Giamundo A., Donati P., De Chiara A. Intracranial Plasma Cell Granuloma. Neurosurgery. 1989;24:591–595. doi: 10.1097/00006123-198904000-00018. [DOI] [PubMed] [Google Scholar]
- 31.Yamataki A., Chiba Y., Tokoro K., Ide K., Yagishita S., Wakabayashi Y., Kan S. Multicentric Intracranial Fibrous Xanthoma—Case Report. Neurol. Med. Chir. 1990;30:759–762. doi: 10.2176/nmc.30.759. [DOI] [PubMed] [Google Scholar]
- 32.Kodsi S.R., Younge B.R., Leavitt J.A., Campbell R.J., Scheithauer B.W. Intracranial Plasma Cell Granuloma Presenting as an Optic Neuropathy. Surv. Ophthalmol. 1993;38:70–74. doi: 10.1016/0039-6257(93)90056-D. [DOI] [PubMed] [Google Scholar]
- 33.Hsiang J., Moorhouse D., Barba D. Multiple Plasma Cell Granulomas of the Central Nervous System: Case Report. Neurosurgery. 1994;35:744–747. doi: 10.1227/00006123-199410000-00024. [DOI] [PubMed] [Google Scholar]
- 34.Le Marc’hadour F., Fransen P., Labat-Moleur F., Passagia J.G., Pasquier B. Intracranial Plasma Cell Granuloma: A Report of Four Cases. Surg. Neurol. 1994;42:481–488. doi: 10.1016/0090-3019(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 35.Makino K., Murakami M., Kitano I., Ushio Y. Primary Intracranial Plasma-Cell Granuloma: A Case Report and Review of the Literature. Surg. Neurol. 1995;43:374–378. doi: 10.1016/0090-3019(95)80067-Q. [DOI] [PubMed] [Google Scholar]
- 36.Breidahl W.H., Robbins P.D., Ives F.J., Wong G. Intracranial Plasma Cell Granuloma. Neuroradiology. 1996;38((Suppl. S1)):S86–S89. doi: 10.1007/BF02278129. [DOI] [PubMed] [Google Scholar]
- 37.Hirohata M., Sasaguri Y., Sugita Y., Tokutomi T., Kobayashi S., Morimatsu M., Shigemori M. Intracranial Plasma-Cell Granuloma: A Case Report. Noshuyo. Byori. 1996;13:133–138. [PubMed] [Google Scholar]
- 38.Saxena A., Sinha S., Tatke M. Intracranial Plasma Cell Granuloma--a Case Report and Review of the Literature. Br. J. Neurosurg. 2000;14:492–495. doi: 10.1080/02688690050175382. [DOI] [PubMed] [Google Scholar]
- 39.Gollogly L., Sadzot B., Lejeune J.P., Deprez M. Meningeal Inflammatory Pseudotumour: A Case Report. Acta. Neurol. Belg. 2001;101:116–120. [PubMed] [Google Scholar]
- 40.Vender J.R., Rupkalvis R., Peterson P., Little B.W., Gennarelli T.A. Extensive Subdural Mass. J. Neuroimaging. 2001;11:76–80. doi: 10.1111/j.1552-6569.2001.tb00016.x. [DOI] [PubMed] [Google Scholar]
- 41.Lacson A., Washington K., Tuite G., Nuttall R. Pathological Case of the Month. Intracranial Plasma Cell Granuloma. Arch. Pediatr. Adolesc. Med. 2001;155:851–852. doi: 10.1001/archpedi.155.7.851. [DOI] [PubMed] [Google Scholar]
- 42.Brandsma D., Jansen G.H., Spliet W., Van Nielen K., Taphoorn M.J.B. The Diagnostic Difficulties of Meningeal and Intracerebral Plasma Cell Granulomas—Presentation of Three Cases. J. Neurol. 2003;250:1302–1306. doi: 10.1007/s00415-003-0200-7. [DOI] [PubMed] [Google Scholar]
- 43.Bigal M.E., Rapoport A.M., Camel M. Cluster Headache as a Manifestation of Intracranial Inflammatory Myofibroblastic Tumour: A Case Report with Pathophysiological Considerations. Cephalalgia. 2003;23:124–128. doi: 10.1046/j.1468-2982.2003.00508.x. [DOI] [PubMed] [Google Scholar]
- 44.Murakami M., Hashimoto N., Kimura S., Hosokawa Y., Kakita K. Intracranial Plasma Cell Granuloma with Genetic Analysis. Acta Neurochir. 2003;145:221–225; discussion 225. doi: 10.1007/s00701-002-1040-4. [DOI] [PubMed] [Google Scholar]
- 45.Buccoliero A.M., Caldarella A., Santucci M., Ammannati F., Mennonna P., Taddei A., Taddei G.L. Plasma Cell Granuloma—An Enigmatic Lesion: Description of an Extensive Intracranial Case and Review of the Literature. Arch. Pathol. Lab. Med. 2003;127:e220–e223. doi: 10.5858/2003-127-e220-PCGEL. [DOI] [PubMed] [Google Scholar]
- 46.Dwarakanath S., Jaiswal A.K., Ralte A.M., Sharma M.C., Mahapatra A.K. Primary Plasma Cell Granuloma of Petrous Bone. J. Clin. Neurosci. 2004;11:552–555. doi: 10.1016/j.jocn.2003.05.012. [DOI] [PubMed] [Google Scholar]
- 47.Shenoy S.N., Raja S. Intracranial Plasma Cell Granuloma. Neurol. India. 2004;52:262. [PubMed] [Google Scholar]
- 48.Shah M.D., McClain K.L. Intracranial Plasma Cell Granuloma: Case Report and Treatment of Recurrence with Methotrexate and 6-Mercaptopurine. J. Pediatr. Hematol. Oncol. 2005;27:599–603. doi: 10.1097/01.mph.0000184636.94923.58. [DOI] [PubMed] [Google Scholar]
- 49.Nawashiro H., Omura T., Kobayashi H. Cystic Intracranial Plasma Cell Granuloma. J. Neurosurg. 2006;105:799–800. doi: 10.3171/jns.2006.105.5.799. [DOI] [PubMed] [Google Scholar]
- 50.Ozüm U., Ozer H., Karadağ O., Polat N. Intracranial Plasma Cell-Granuloma with Extensive Ossification. Br. J. Neurosurg. 2006;20:153–156. doi: 10.1080/02688690600776978. [DOI] [PubMed] [Google Scholar]
- 51.Flannery T., Al-Sabah F., Bhangu J., Alderazi Y., Brett F., Pidgeon C. Treatment of Subtotally Resected Intracranial Plasma Cell Granuloma with Steroids: A Case Report. Br. J. Neurosurg. 2007;21:501–503. doi: 10.1080/02688690701398706. [DOI] [PubMed] [Google Scholar]
- 52.Brito C.C.B., Lopes F.C.R., Chimelli L., Gasparetto E.L. Intracranial Cell Plasma Granuloma. Arq. Neuropsiquiatr. 2010;68:127–129. doi: 10.1590/S0004-282X2010000100026. [DOI] [PubMed] [Google Scholar]
- 53.Chen D., Liu L., Qiu L. The MRI Misdiagnosis Analysis of Intracranial Inflammatory Myofibroblastic Tumor (4 Cases Report and Literature Review) J. Clin. Radiol. 2009;3:42. [Google Scholar]
- 54.Puntambekar P., Santhakumar S., Kupsky W.J., Tselis A., Mittal S. Primary Intracranial Plasma Cell Granulomas Presenting as Malignant Neoplasms. J. Neurooncol. 2012;106:327–337. doi: 10.1007/s11060-011-0667-5. [DOI] [PubMed] [Google Scholar]
- 55.Kato K., Moteki Y., Nakagawa M., Kadoyama S., Ujiie H. Inflammatory Myofibroblastic Tumor of the Cerebellar Hemisphere—Case Report. Neurol. Med. Chir. 2011;51:79–81. doi: 10.2176/nmc.51.79. [DOI] [PubMed] [Google Scholar]
- 56.Fan F., Lei C., Dong-Liang L., Peng S. Inflammatory Myofibroblastic Tumor Mimicking Malignant Meningioma in the Middle Cranial Fossa: A Case Report. Chin. Med. Sci. J. 2012;27:185–187. doi: 10.1016/S1001-9294(14)60054-7. [DOI] [PubMed] [Google Scholar]
- 57.Wang J., Sun Z., Zhuo S., Wang K. Sigmoid Sinus Occlusion Infiltrated by Inflammatory Myofibroblastic Tumor from Mastoid. Head Neck. 2015;37:E4–E7. doi: 10.1002/hed.23704. [DOI] [PubMed] [Google Scholar]
- 58.Chuah Y.-Y., Tashi T., Shy C.-G., Shyu J.-S., Dong M.-J., Hsueh E.-J. Intracranial Inflammatory Myofibroblastic Tumor with Sarcomatous Local Recurrence. World Neurosurg. 2016;93:484. e1–484. e4. doi: 10.1016/j.wneu.2016.07.060. [DOI] [PubMed] [Google Scholar]
- 59.Lata K.A., Upadhyay V., Pawar S.S. A Rare Case of Inflammatory Myofibroblastic Tumor of Meninges. J. Pediatr. Neurosci. 2018;13:255–259. doi: 10.4103/JPN.JPN_117_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Malnik S.L., Moor R.F., Shin D., Laurent D., Trejo-Lopez J., Dodd W., Yachnis A., Ghiaseddin A.P., Fox W.C., Roper S. Inflammatory Myofibroblastic Tumor Masquerading as an Anterior Choroidal Artery Fusiform Aneurysm. Surg. Neurol. Int. 2021;12:297. doi: 10.25259/SNI_113_2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All of the data in this study can be found in the PubMed database.