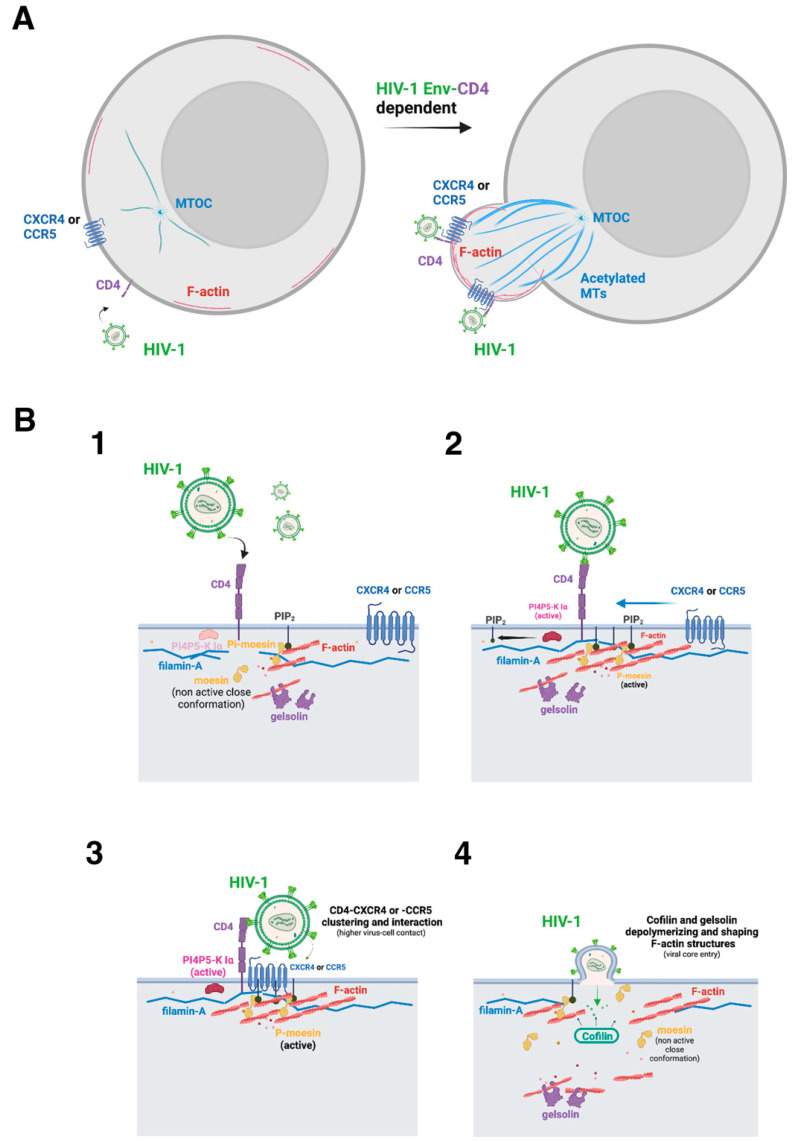
Figure 2.
Scheme illustrating the events associated with cell actin cytoskeleton reorganization that underlie HIV-1 Env function to drive pore fusion formation, viral entry, and infection. (A) Functional HIV-1 Env binds to lymphocyte CD4 to signal activating actin-associated factors that reorganize cortical microfilaments to create a pseudopod “hot zone” where viral receptors aggregate and the fusion pore is formed, thus initiating the infection process, as represented in the next panel. (B) The illustration represents the main molecular events reported to be involved in HIV-1 Env-mediated pore fusion formation, viral entry, and infection that occurred at the pseudopod structure (panel (A)). The first HIV-1 Env/CD4 interaction (1), through the viral gp120 subunit, activates the cytoplasmic dormant actin-adaptor moesin, which is phosphorylated in its actin-binding C-terminal domain, thus favoring F-actin polymerization and anchoring cortical AFs to the inner leaflet of the plasma membrane either directly by its association with PIP2 or through several receptors (2). HIV-1 Env/CD4 binding also activates the PI4P5-K Iα kinase that produces PIP2 (2). This PIP2 molecule could mediate the plasma membrane/moesin/AFs link by recruiting the N-terminal domain of moesin (2 and 3). These events polymerize and reorganize the actin cytoskeleton around the virus–cell contact regions (3). Filamin-A stabilizes these actin areas by anchoring AFs with the cytoplasmic domains of the HIV-1 CD4, CXCR4, and CCR5 receptors (3). Therefore, filamin-A and moesin concentrate viral receptors in F-actin capping areas. These cortical AFs should be shaped in size by the actin-severing protein gelsolin to be reorganized and alter cell surface dynamics (4), generating a pseudopod region (see panel (A)) where the actin and tubulin cytoskeleton, their associated factors and viral receptors, aggregate and interact. It is thought that this capping region or “hot zone” generated by HIV-1 Env increases the probability of the virus recognizing its receptors and promoting the formation of the fusion pore (i.e., membrane–lipid exchange) by linking cortical AFs with the cell surface at regions where HIV-1 Env anchors (i.e., through the gp41 fusion protein). The actin-disrupting cofilin factor depolymerizes the cortical node created to form the fusion pore to permit the HIV-1 capsid to enter cells and encounter the tubulin cytoskeleton to travel to the nucleus (4). This step is also favored by the dephosphorylation of the viral activated moesin that releases AFs from their attachment to the plasma membrane (4). Designs and templates were created with BioRender.