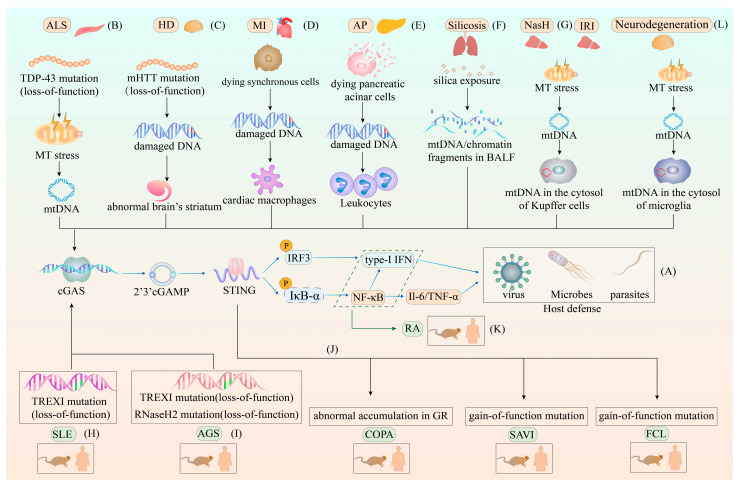
Figure 3.
The different roles of the cGAS–STING pathway in host defense and various diseases. (A) The role of the cGAS–STING pathway in host defense. The genome of invading pathogens, such as viruses, bacteria and parasites, in the cytosol can be sensed by cGAS, triggering the cGAS–STING pathway-induced production of type-I IFN and other cytokines to eliminate the genomes of invading pathogens. (B) The role of the cGAS–STING pathway in ALS. The missense mutation in the TDP-43 protein enables its absorption into the mitochondria and causes mtDNA leakage into the cytosol, prompting overproduction of type-I IFN and NF-κB to initiate hyperinflammatory responses. (C) The role of the cGAS–STING pathway in HD. The mutation of the N-terminal polyglutamine in the huntingtin protein (mHTT) leads to the abnormal accumulation of damaged DNA in the brain’s striatum, initiating the STING-dependent type-I IFN production and autophagy. (D) The role of the cGAS–STING pathway in MI. Synchronously dying cells are sensed by cardiac macrophages, and damaged DNA from these cells is internalized by cardiac macrophages. Furthermore, this internalization process leads to the inhibition of the transformation of cardiac macrophages from inflammatory cells to a reparative phenotype via the activation of the cGAS–STING pathway. (E) The role of the cGAS–STING pathway in AP. Damaged DNA released by the dying pancreatic acinar cells is internalized by leukocytes, resulting in severe inflammation due to the cGAS–STING pathway-induced overproduction of type-I IFN and other cytokines. (F) The role of the cGAS–STING pathway in silicosis. Silica microparticles invade the lungs, causing an increased release of chromatin fragments and mtDNA from dying cells into the BALF. These chromatin fragments and mtDNA then engage the cGAS–STING pathway to increase type-I IFN and other cytokine levels, causing chronic progressive fibrotic inflammation in the lungs. (G) The role of the cGAS–STING pathway in NASH and liver IRI. Lipotoxicity in NASH and ROS in IRI both lead to mitochondrial stress and the release of mtDNA into the cytosol of Kupffer cells. Furthermore, these changes result in adipose tissue inflammation via the cGAS–STING pathway and cGAS–STING–NLRP3 inflammasome-driven cell death in NASH and IRI, respectively. (H) The role of the cGAS–STING pathway in SLE. The loss-of-function mutation in TREX1 is suggested as the main cause of SLE. TREXI mutations lead to the abnormal accumulation of cytosolic DNA, which continuously activates the type-I IFN signaling cascade and amplifies inflammation via cGAS–STING. (I) The role of the cGAS–STING pathway in AGS. The loss-of-function mutation in TREX1 and the RNaseH2 complex are considered the major components of AGS. In this context, mutations in TREX1 and the RNaseH2 complex lead to the failure to eliminate genomic fragments in the cytosol and maintain genome stability, causing increased cGAS–STING pathway-dependent expression of type-I IFN. (J) The role of the cGAS–STING pathway in COPA, SAVI, and FCL. COPA is caused by the abnormal accumulation of STING in the GR. SAVI is attributed to the gain-of-function mutation in the STING-encoding gene, which leads to the spontaneous translocation of STING from the ER to the GR. This abnormal translocation activates intensive type-I IFN signals independently of cGAMP stimulation. Finally, FCL is caused by the heterozygous gain-of-function mutation in STING, resulting in the spontaneous dimerization of STING and constitutive activation of the type-I IFN signature. (K) The role of the cGAS–STING pathway in RA. The abnormal accumulation of cytosolic DNA leads to the expression of cytokines and chemokines via cGAS–STING activation, contributing to RA development. (L) The role of the cGAS–STING pathway in neurodegeneration. In microglia, mtDNA in the cytosol of microglia contributes to the chronic inflammatory response, which leads to neurodegeneration. Abbreviations: ALS, amyotrophic lateral sclerosis; MT stress, mitochondrial stress; mt DNA, mitochondrial DNA; TDP-43 protein, TAR DNA-binding protein 43; HD, Huntington disease; mHTT, mutation of the N-terminal polyglutamine in huntingtin protein; MI, myocardial infarction; AP, acute pancreatitis; BALF, bronchoalveolar lavage fluid; NASH, nonalcoholic steatohepatitis; IRI, ischemia-reperfusion injury; SLE, systemic lupus erythematosus; AGS, Aicardi–Goutières syndrome; COPA, COPA syndrome; SAVI, STING-associated vasculopathy with infantile-onset; FCL, familial chilblain lupus; RA, rheumatoid arthritis; cGAS–STING, cyclic GMP-AMP synthase-stimulator of interferon genes; type-I IFN, type-I interferon.