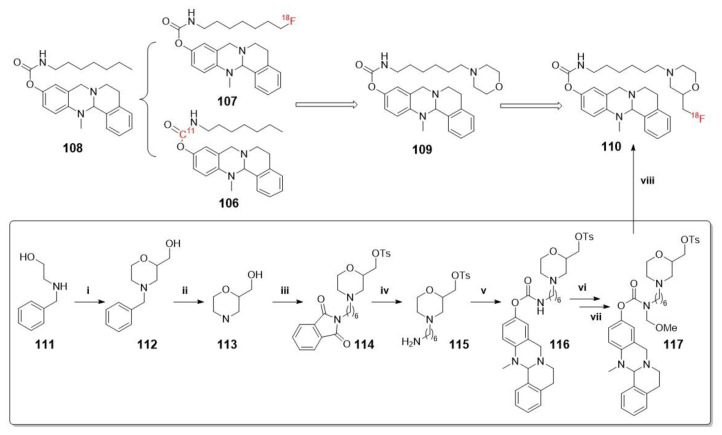
Scheme 29.
Synthesis of 110. Reagents and conditions: (i) 1. epichlorohydrine, RT, 3 h; 2. H2SO4, 140 °C, 1 h; 3. H2O, CHONH2, 145 °C, 20 h; (ii) H2, Pd/C, MeOH, RT, 1 h; (iii) 1. 2-(6-bromohexyl)isoindoline-1,3-dione, N,N-diisopropylethylamine (DIPEA), DMF, 100 °C, 20 h; 2. TosCl, NEt3, CH2Cl2, RT, 20 h; (iv) H4N2·H2O, EtOH, 80 °C, 1.5 h; (v) 1. p-nitrophenyl chloroformate, NEt3, CH2Cl2, 3 h, RT; 2. 13-methyl-5,8,13,13a-tetrahydro-6H-isoquinolino [1,2-b]quinazolin-10-ol, NaH, CH2Cl2, 2 h, RT; (vi) TMS Cl, para-formaldehyde, CH2Cl2, RT, 18 h; (vii) MeOH, RT, 1 h; (viii) 1. [ 18F]KF, K222, MeCN, 110 °C, 10 min; 2. 6 M HClaq, 90 °C, 5 min.