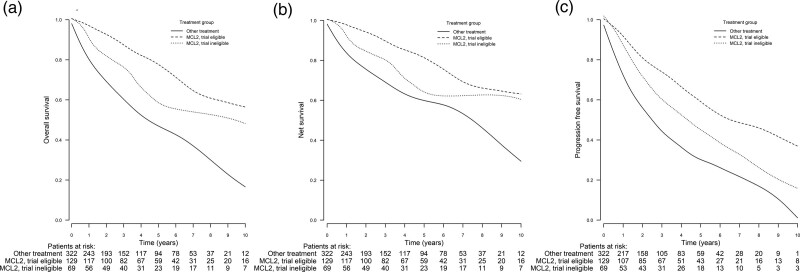
Figure 1.
Comparison of 2-, 5-, and 10-year OS, NS, and PFS of the MCL2-treated trial-eligible and trial-ineligible groups and the other group, who did not receive MCL2 treatment. (A) OS at 2, 5, and 10 years. The trial-eligible group: 93% (95% CI, 89-98), 79% (95% CI, 71-87), and 57% (95% CI, 45-72). The trial-ineligible group: 84% (95% CI, 75-94), 59% (95% CI, 46-74), and 49% (95% CI, 34-70). The other group: 70% (95% CI, 65-75), 47% (95% CI, 41-53), and 17% (95% CI, 11-25). The OS was significantly improved in the trial-eligible and trial-ineligible groups compared with the other group (P < 0.001 in both comparisons). The median OS in the trial-eligible group was also improved compared with the trial-ineligible group (P < 0.001). (B) NS at 2, 5, and 10 years. The trial-eligible group: 95% (95% CI, 90-99), 82% (95% CI, 74-91), and 64% (95% CI, 51-80). The trial-ineligible group: 86% (95% CI, 77-96), 64% (95% CI, 51-81), and 61% (95% CI, 43-87). The other group: 76% (95% CI, 71-82), 60% (95% CI, 53-69), and 29% (95% CI, 20-44). NS was significantly improved in the trial-eligible and trial-ineligible groups compared with the other group (P < 0.001 in both comparisons). There was no statistically significant difference between the trial-eligible and trial-ineligible groups (P = 0.368). (C) PFS at 2, 5, and 10 years. The trial-eligible group: 81% (95% CI, 74-89), 62% (95% CI, 53-73), and 40% (95% CI, 29-56). The trial-ineligible group: 72% (95% CI, 62-85), 46% (95% CI, 34-62), and 23% (95% CI, 11-45). The other group: 57% (95% CI, 51-63), 30% (95% CI, 25-37), and 2% (95% CI, 0-13). PFS was significantly improved both in the trial-eligible group (P < 0.001) and trial-ineligible (P = 0.003) group compared with the other group. The median PFS in the trial-eligible group was also improved compared with the trial-ineligible group (P = 0.030). CI = confidence interval; MCL = mantle cell lymphoma; NS = net survival; OS = overall survival; PFS = progression-free survival.