Table 1.
Results of the reactions of resorcinols and naphthols with citronellal a.
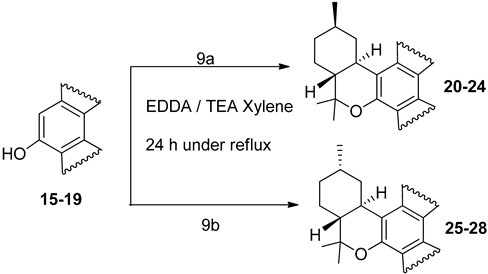
| ||||
| Entry | Starting Material | Citronellal | Product | Yield (%) |
| 1 |
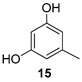
|
9a |
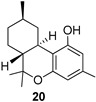
|
68 |
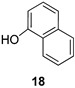
| 2 |
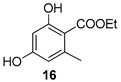
|
9a |
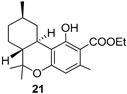
|
87 |
| 3 |
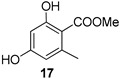
|
9a |
|
75 |
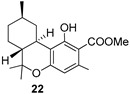
| 3 |
|
9a |
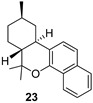
|
92 |
| 4 |
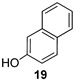
|
9a |
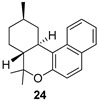
|
72 |
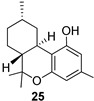
| 5 |
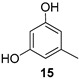
|
9b |
|
70 |
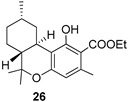
| 6 |
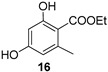
|
9b |
|
87 |
| 7 |
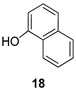
|
9b |
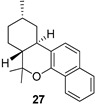
|
90 |
| 8 |
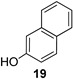
|
9b |
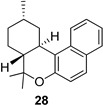
|
75 |
a Reaction conditions: starting material (1.0 mmol), citronellal (1.5 mmol), EDDA (20% mol), TEA (0.2% mol) in xylene [21].
