Figure 3. Cryo-EM structure of N751-2C09.01 in complex with BG505 DS-SOSIP reveals epitope similarity to N751-2C06.01.
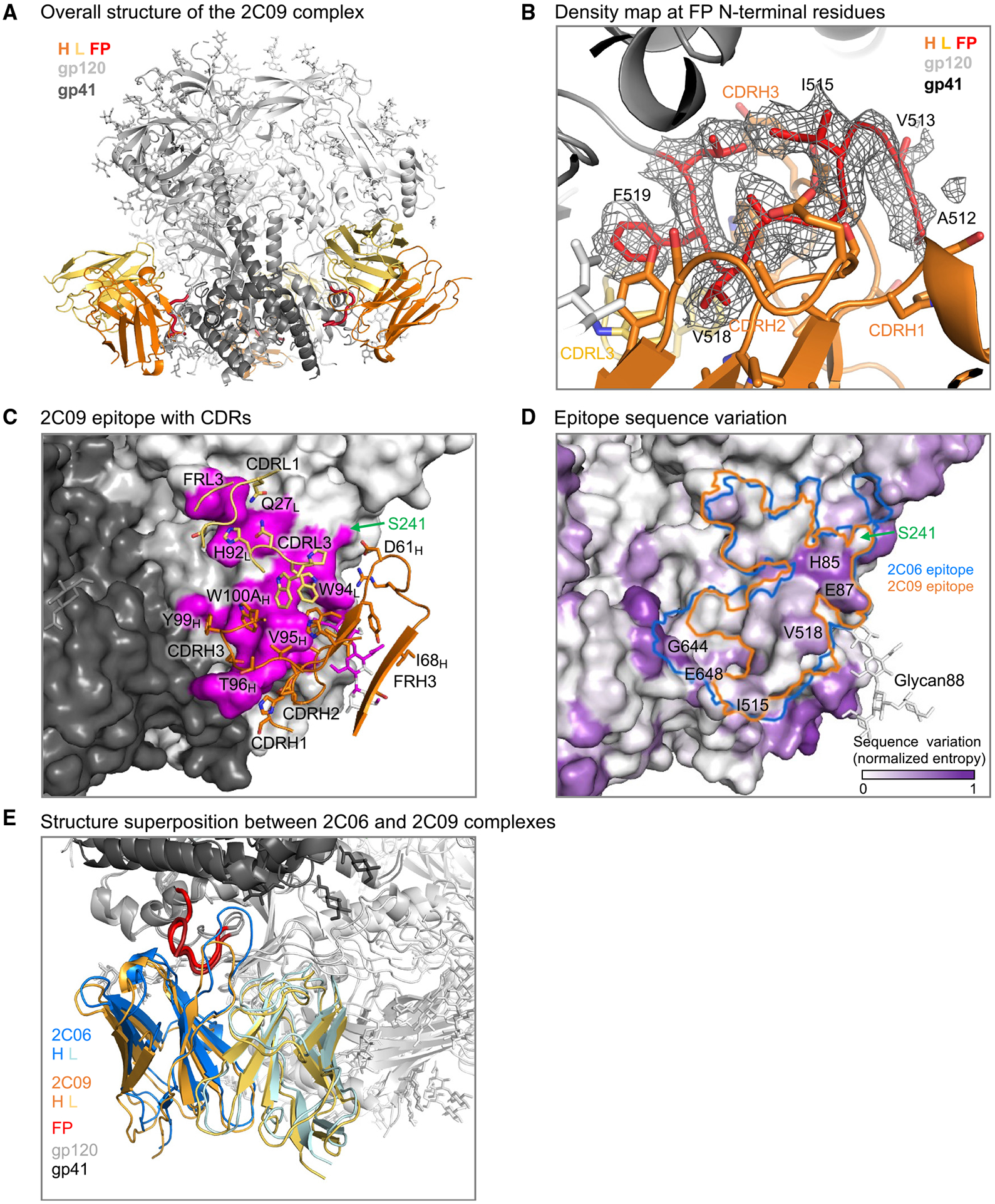
(A) Cartoon representation of 2C09 Fab in complex with BG505 DS-SOSIP. 2C09 bound at the fusion-peptide site and interacted with fusion peptide extensively. Heavy chain (H) is shown in orange, light chain (L) in yellow-orange, fusion peptide (FP) in red, the rest of gp41 in darker gray, and gp120 in lighter gray.
(B) Density map showing well-ordered density for fusion peptide. The structure is shown in cartoon representation, with side chains of fusion-peptide residues 512–520 shown in sticks. Some side chains of the antibody interacting with fusion peptide are also shown in sticks.
(C) Epitope details of antibody 2C09 shown for one of the binding sites on the trimer. Env trimer is shown as surface, with subunits in different shades of gray and the epitope surface in magenta. Antibody CDRs and FRs involved in binding are shown in cartoon representation, with interacting side chains shown in sticks. The glycan hole at S241 is on the edge of the epitope and is labeled.
(D) Sequence conservation around the 2C09 epitope. Normalized sequence entropy of HIV-1 group M is mapped on the trimer surface, with absolutely conserved residues (entropy of 0) in white and the most diverse residues (entropy of 1) in purple. 2C09 epitope is shown as an orange outline. The outline of 2C06 is shown in blue for comparison.
(E) Structure superposition between 2C06 and 2C09 complexes. The two structures were aligned by the gp41 subunit bearing the fusion peptide shown. The fusion peptide in both structures were in similar conformation except the first two residues, which in 2C09 (darker red) folded back down to interact with the antibody and in 2C06 extended up to bind the C-terminal helix of neighboring gp41 (top left).
